A total of 334 end-stage renal disease patients with moderate-to-severe uraemic xerosis were surveyed for quality of life assessment, using the generic Short-Form (SF-12) scale and the Dermatology Life Quality Index (DLQI). In parallel, the intensity of xerosis at four sites (the two lower legs, chest, forearm without arterio-venous shunt) was assessed, using a five-point lesional intensity score. Pruritus was auto-assessed by the patients, using a 100-mm visual analogue scale. Uraemic xerosis patients had a marked deterioration in the Physical Component Summary of SF-12 (mean ± SD: 34.92±9.98) and DLQI (5.06 ± 4.73). Younger age (r = –0.20), xerosis intensity (r = 0.14), and the presence of pruritus (p < 0.0001) and its intensity (r = 0.50) were shown to be significant worsening factors of DLQI. Because a low, but significant, correlation between the intensity of xerosis and pruritus was also demonstrated (r = 0.18), the direct contribution of age, xerosis and pruritus on DLQI was analysed in a multiple linear regression model. Age and pruritus intensity, but not xerosis intensity, were found to be independent contributors to DLQI deterioration (p < 0.0005). On the other hand, uraemic xerosis without associated pruritus still resulted in DLQI alteration (3.24 ± 3.99). It was concluded that young age and intensity of uraemic pruritus compromise quality of life in uraemic xerosis patients. Some characteristics of uraemic xerosis other than xerosis intensity may also be involved in quality of life alteration. Key words: uraemic xerosis; uraemic pruritus; quality of life; DLQI; SF-12.
(Accepted November 15, 2010.)
Acta Derm Venereol 2011; 91: XX–XX.
Patrick Dupuy, Orfagen, CRDPF Langlade, 3 Avenue Hubert Curien, FR-31035 Toulouse, France. E-mail: patrick.dupuy@orfagen.com
Xerosis (rough and scaly skin) affecting patients under maintenance renal dialysis (MRD) is a poorly recognized entity that was described previously as “acquired ichthyosis” (1), and more recently by the preferred term “uraemic xerosis” (2). It is a neglected disease, with little clinical research. However, it becomes a prominent feature after patients with end-stage renal disease (ESRD) start haemodialysis or peritoneal dialysis. Uraemic xerosis has also been described as being an important factor influencing uraemic pruritus (3–6), although the instrumentally measured water content of the stratum corneum (corneometry) does not correlate with pruritus intensity (7). In published series, xerosis of moderate to severe intensity led to a 50–100% increase in uraemic pruritus (4–6, 8). It was hypothesized that uraemic xerosis, even if it is not the primary cause of pruritus, has a worsening effect by reducing the threshold for itch (9). Both uraemic xerosis and pruritus may also result in aggravated skin excoriation, prurigo nodularis and infections (8). Uraemic pruritus itself is frequent and leads to marked suffering and distress in MRD patients (10–12), as itchy patients in general experience psychosocial burdens (12, 13).
The psychological and social consequences of uraemic xerosis have not been published. As a chronic and widely distributed condition, its physical and emotional impact appears largely to be underestimated in clinical practice. The aim of this study was to provide a precise description of the quality of life (QoL) status of patients with moderate-to-severe uraemic xerosis. Correlation tests on QoL with uraemic pruritus were also investigated. The study was part of therapeutic trials (manuscripts in preparation), of which the baseline conditions are presented here.
METHODS
The study took advantage of the enrolment of 334 patients distributed over two therapeutic trials in Europe (Czech Republic, n = 9; France, n = 57; Germany, n = 56; Greece, n = 65; Hungary, n = 19; Italy, n = 24; Poland, n = 104) in order to assess their baseline QoL status. The enrolment period ranged from September 2003 to November 2008, during which patients undergoing MRD in the participating dialysis centres were systematically reviewed for selection for the study according to specific inclusion and exclusion criteria. The two therapeutic trials had similar inclusion and exclusion criteria. Adult patients (at least 18 years of age) of both sexes undergoing haemodialysis or peritoneal dialysis because of ESRD were studied. All patients had a clinical diagnosis of uraemic xerosis, i.e. a skin xerosis related to their MRD status, whereas those with skin complications (prurigo, superinfection, contact dermatitis) were excluded. Patients were examined by a dermatologist to evaluate disease intensity, using the El-Gammal severity score (14) on the following body sites: chest, forearm without arteriovenous shunt and the two lower legs. The El Gammal index includes five items: 0 = smooth skin; 1 = patches of fine, powdery scales; 2 = diffuse ashy appearance with many fine scales; 3 = moderate scaling with beginning cracks; and 4 = intense scaling, moderate cracks. In order to minimize inter-assessor variability, a photograder illustrating each grade was provided. Only patients with a score of 2 or more on at least one site (moderate to severe xerosis) were included. A total score was deduced for each patient by summing the scores of each site. Global intensity of uraemic pruritus was assessed by the patients, using a 100-mm visual analogue scale (VAS; from 0 = no itch at all, to 10 = extreme itch). Anti-xerotic treatments (moisturizing emollients) were stopped at least one week before study assessment. Oral antihistamines were maintained at stable doses for at least 2 weeks, and phototherapy was discontinued for at least 3 weeks before data collection.
QoL was evaluated by the patients by means of two different scales: the Short-Form 12 (SF-12) and the Dermatology Life Quality Index (DLQI). SF-12 is a generic index, with multipurpose measurements of health status. It was developed as a shorter, yet valid, alternative to SF-36 for use in large surveys of general and specific populations (15). It measures eight concepts commonly represented in widely used surveys, and comprises 12 items: physical functioning (2 items), role-physical (2 items), bodily pain (1 item), general health (1 item), energy/fatigue (1 item), social functioning (1 item), role-emotional (2 items) and mental health (2 items). It can be analysed through two components, the Physical Component Summary (PCS-12) and the Mental Component Summary (MCS-12). Both PCS-12 and MCS-12 are scored using norm-based methods. PCS-12 and MCS-12 are transformed to have a mean ± SD of 50 ± 10 in the general US population (16). The lower the scores, the more the components are altered. The advantage of standardization and norm-based scoring is that results can be meaningfully compared with other conditions, bearing in mind that the distribution of scores with European patients can be interpreted only in relation to the US population.
DLQI is a specific scale assessing the impact of dermatological diseases on patient quality of life (17). It is self-explanatory and easily handled by the patients. It comprises six concepts and 10 items: symptoms and feelings (2 items), daily activities (2 items), leisure (2 items), work and school (1 item), personal relationships (2 items) and treatment (1 item). It is calculated by summing the score of each item (graded from 0 to 3), resulting in a minimum of 0 and a maximum of 30. The higher the score, the more QoL is compromised. It is the most commonly used instrument for QoL evaluation in dermatology, but may not be very sensitive to detect small impairments (9).
For each individual, SF-12 and DLQI questionnaires were given as separate sheets in an envelope that was sealed after being completed. All the questionnaires were provided in local languages (validated language versions). Mean values ± SD were calculated for all scores.
The influence of certain variables on DLQI was studied including categorical variables such as sex, ESRD underlying disease, the type of MRD, and the presence or not of pruritus. Continuous variables were the patient’s age, the duration of MRD, the duration of xerosis and pruritus, and the clinical intensity of uraemic xerosis (El Gammal total score) and uraemic pruritus (VAS).
Statistical comparison of the DLQI results between stratified subpopulations according to categorical variables was made using the Student’s t-test. Correlation analysis between continuous variables and QoL scores was performed using Pearson’s correlation test. The respective contribution of the factors of age, xerosis intensity and pruritus intensity on the scores were studied using a multiple linear regression analysis.
The study protocols and data collection procedure were approved by local or national ethics committees. Written informed consent was obtained from all patients.
RESULTS
A total of 334 patients were invited to participate in the study. Seven (7) patients (2%) declined or failed to complete the SF-12 and DLQI questionnaires. Finally, data were available for 306 patients for SF-12 analysis (92%) and for 327 patients for DLQI analysis (98%). The characteristics of the study population (n = 334) are given in Table I.
Table I. Mean demographic features and lesional skin status of the study population
|
Variables
|
n = 334
|
|
Age, years, mean ± SD
|
64.21 ± 0.72
|
|
Gender, n (%)
|
|
|
Male
|
187 (56)
|
|
Female
|
147 (44)
|
|
ESRD underlying disease, n (%)
|
|
|
Glomerular diseases
|
90 (27)
|
|
Diabetic nephropathy
|
68 (20)
|
|
Nephrosclerosis
|
71 (21)
|
|
Polycystic kidney
|
30 (9)
|
|
Pyelonephritis
|
25 (7)
|
|
Others
|
58 (17)
|
|
Unknown
|
15 (4)
|
|
Type of MRD, n (%)
|
|
|
Haemodialysis
|
321 (96)
|
|
Peritoneal dialysis
|
13 (4)
|
|
Duration of MRD, years, mean ± SD
|
5.61 ± 0.31
|
|
Duration of xerosis, years, mean ± SD
|
4.65 ± 0.25
|
|
Intensity of xerosis, total score, mean ± SD
|
7.88 ± 0.14
|
|
Association with pruritus, n (%)
|
|
|
Yes
|
221 (66)
|
|
No
|
113 (34)
|
|
Duration of pruritus, years, mean ± SD
|
4.29 ± 0.29
|
|
Intensity of pruritus, VAS, mm, mean ± SD
|
38.02 ± 1.85
|
SD: standard deviation; ESRD: end-stage renal disease; MRD: maintenance renal dialysis; VAS: visual analogue scale.
Whereas the MCS component of SF-12 was slightly decreased (mean ± SD: 43.81 ± 11.78), its PCS component was severely impaired (34.92 ± 9.98). Similarly, the DLQI measurement was markedly compromised (5.06 ± 4.73). According to the individual score classification proposed by Hongbo et al. (18), DLQI distribution among the study population (n = 327) was as follows: 92 patients with an index of less than 2 (28%), 103 patients between 2 and 5 (33%), 67 patients between 6 and 10 (21%) and 69 patients with more than 10 (18%). The main contributing factors to DLQI alteration were the symptoms and feelings items (41%) and, to a lesser extent, the daily activities (20%).
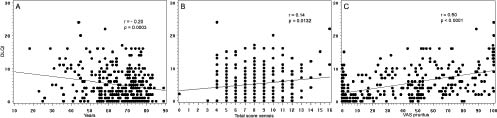
DLQI results according to demographic variables and disease-related factors are given in Table II for the categorical variables and in Fig. 1 for certain continuous variables. Among the demographic variables, only age was found to significantly interfere in a negative manner in the DLQI, i.e. with more severe scores for patients who were younger (r = –0.20, p = 0.0003; Fig. 1a). The gender, ESRD causal disease, type of MRD and duration of MRD had no significant impact on DLQI (Table II). Moreover, lesional skin parameters, such as duration of xerosis and pruritus, were not shown to modify significantly the DLQI score (data not shown). By contrast, the presence of pruritus was clearly associated with a more severe DLQI (p < 0.0001; Table II). Similarly, the clinical intensity of xerosis was a weak but significant prognostic factor for DLQI worsening (r = 0.14, p = 0.013; Fig. 1b), and a significant correlation between the intensity of pruritus and the impairment of DLQI (r = 0.50, p < 0.0001; Fig. 1c) was also demonstrated.
Table II. Dermatology Life Quality Index (DLQI) scores in categorical demographic and lesional subpopulations of the study
|
Subpopulations
|
DLQI
Mean ± SD
|
p-value
|
|
Gender
|
|
NS
|
|
Male
|
4.91 ± 4.77
|
|
Female
|
5.24 ± 4.70
|
|
MRD causal disease
|
|
NS
|
|
Glomerular diseases
|
4.72 ± 4.90
|
|
Diabetic nephropathy
|
5.32 ± 4.66
|
|
Nephrosclerosis
|
5.54 ± 5.20
|
|
Polycystic kidney
|
4.90 ± 4.58
|
|
Pyelonephritis
|
5.44 ± 5.00
|
|
Others
|
4.82 ± 3.78
|
|
Unknown
|
6.57 ± 5.47
|
|
Type of MRD
|
|
NS
|
|
Haemodialysis
|
5.12 ± 4.78
|
|
Peritoneal dialysis
|
3.18 ± 2.56
|
|
Association with pruritus
|
|
<0.0001
|
|
Yes
|
5.99 ± 4.82
|
|
No
|
3.24 ± 3.99
|
SD: standard deviation; MRD: maintenance renal dialysis.
Fig. 1. Dermatology Life Quality Index (DLQI) scores according to (A) age, (B) xerosis intensity (total score), and (C) pruritus intensity (visual analogue scale; VAS) in the study population. Correlation analyses were performed using Pearson’s correlation test.
On the other hand, a low but significant positive correlation between xerosis intensity and pruritus intensity was demonstrated, i.e. pruritus increased significantly when xerosis was more intense (r = 0.18, p = 0.001; Fig. 2). By contrast, there was no significant correlation between age and xerosis or between age and pruritus. In order to investigate any possible confounding effects between age, xerosis and pruritus on QoL impairment, the direct contribution of the respective factors on DLQI was analysed using a multiple linear regression model with age, pruritus intensity and xerosis intensity as variables. Taken together, the three variables were found to be significant contributors to the DLQI scores (R2 = 0.28, p < 0.0001). Age (β = –0.17, p = 0.0004) and pruritus intensity (β = 0.47, p < 0.0001) had an independent effect on the DLQI score, whereas lesional intensity of xerosis as measured by total lesional score (El Gammal score) had no significant direct effect on DLQI (β = 0.05, p = 0.30). Partial correlation analysis confirmed that the xerosis intensity without the influence of pruritus was not a significant contributing factor to DLQI alteration (p = 0.47). However, DLQI in patients without associated pruritus remained compromised (3.24 ± 3.99; Table II).

Fig. 2. Correlation analysis between intensity of xerosis (total score) and intensity of pruritus (visual analogue scale; VAS) in the study population, using Pearson’s correlation test.
DISCUSSION
This study is the first survey investigating the QoL of patients with uraemic xerosis. Uraemic xerosis is a common chronic cutaneous complication among ESRD patients undergoing MRD (3, 19). According to the literature, it affects 50–85% of MRD patients (4, 6, 8), whereas 30–40% of ESRD patients report this symptom before starting MRD (4, 20). Furthermore, the majority of uraemic xerosis cases observe remission of the xerotic signs after renal transplantation.
Uraemic xerosis often affects the entire surface of the body, and may be more intense in some areas. In large series, the intensity of the lesions has been described as mild in 30–40%, moderate in 35–50%, and severe in 15–30% of MRD patients (4, 6). It is a permanent syndrome, with a clinical picture comprising a dry skin appearance, marked scaling and roughness, and poor skin turgor (i.e. failure of the skin to reassume a prompt normal contour when the skin is stretched). Associated signs are premature skin ageing (elastosis) and pruritus (3, 21). Severe involvement of certain areas, such as the hands and feet, leads to possible functional impairment. Because the cutaneous barrier function is reduced, the skin is more easily exposed to external attacks and aggression, such as wind, cold, sun and reduced air humidity (22). As in some other severe xerotic conditions, a greater susceptibility to irritation caused by chemical factors (e.g. soaps and detergents) may be observed (23). Therefore, patients should be advised to avoid frequent hand-washing and baths in order to limit cumulative soap-induced irritation (24). Irritative clothes must often be replaced by smoother fabrics (e.g. cotton; 25). In some patients, uraemic xerosis is associated with diminished sweating and poor wound healing (26–28). The cause of uraemic xerosis is unknown. The skin is a major reservoir of water, containing 10–20% of the total body water content (22); it is conceivable that MRD sessions, where the volemia equilibrium is frequently disturbed, requires water homeostasis at the expense of cutaneous integrity at the epidermal and even the dermal level (e.g. elastin disruption).
This study used validated cross-cultural QoL questionnaires (SF-12, DLQI). In the study population, which experienced both ESRD and uraemic xerosis, overall health-status-related QoL impairment and skin-related QoL impairment were analysed using, respectively, SF-12 and DLQI items. It was found using the generic SF-12 questionnaire that ESRD patients undergoing MRD have a significant alteration in their QoL, with a reduction prevailing on its physical component (mean ± SD PCS: 34.92 ± 9.98). The dermatologically orientated DLQI questionnaire demonstrated that uraemic xerosis is partly the cause of their QoL aggravation (5.06 ± 4.73). By ranking the degree of QoL alteration according to individual DLQI results (18), uraemic xerosis patients actually distributed widely from no impairment (DLQI < 2) to very strong QoL impairment (DLQI > 11). Patients with complications of their xerosis (e.g. prurigo, irritant dermatitis, skin infection), who were not included in this study, may have deeper QoL alteration. Uraemic xerosis affects QoL through the bad feelings and low self-esteem the symptoms induce; however, the physical component and daily activity outcome also participated in QoL alteration.
Analysis of demographic and lesional status variables on DLQI revealed that younger patients with uraemic xerosis suffer more than those of older age (r = –20; p = 0.0003). Hypothesizing that these patients represent the most economically active ESRD subpopulation, this suggests that the condition may also have an aggravating socio-economical impact. The sex, personal history (ESRD causal condition, duration of MRD, xerosis and pruritus) and MRD modality were not contributive to their QoL alteration. In our study, two-thirds of the uraemic xerosis patients had persisting pruritus despite the fact that antihistamine treatments were allowed, whereas an increased sensitivity to histamine has been reported previously in patients with uraemic pruritus (29). The presence of pruritus resulted in greater QoL alteration (p < 0.0001). A previous study using SF-36 and DLQI also showed markedly decreased QoL in MRD patients with uraemic pruritus compared with those who were free from itch (30).
Both uraemic xerosis intensity and pruritus intensity were apparently shown to have a negative impact on QoL (r = 0.14 and r = 0.50, respectively; p < 0.02), but a positive correlation between the two variables (r = 0.18, p = 0.001) was also demonstrated. Our findings confirmed the previous observations that uraemic xerosis aggravates uraemic pruritus (4, 6), whereas there was no interaction between age and xerosis or age and pruritus. Such a correlation raises the possibility that xerosis may be a confounding factor. Using a multiple linear regression model, we indeed found that age and pruritus intensity taken individually both compromised QoL (p < 0.0005), but xerosis intensity had no distinct impact on QoL. We can deduce that the intensity of xerotic lesions, as measured by the El Gammal score, compromise the QoL of uraemic xerosis patients mainly by aggravating the associated pruritus. Nevertheless, uraemic xerosis patients without associated pruritus also had QoL impairment, but to a lesser extent (mean ± SD DLQI: 3.24 ± 3.99). Other xerosis-related variables not taken into account in our xerosis assessment may also be direct contributors to QoL alteration, for instance the extensive spreading of xerotic lesions that was observed in most patients or the intensity of the lesions on the hands and feet that were not accounted as test sites. The chronicity of xerosis, as another possible factor, was in fact tested (e.g. duration of xerosis) but not found to affect QoL.
In conclusion, our data clearly demonstrate that ESRD patients under MRD experience a severe reduction in their QoL. Uraemic xerosis and uraemic pruritus, which participate in the deterioration in their QoL, have a psychosocial impact that appears to be largely underestimated in clinical practice. Uraemic xerosis compromises QoL indirectly by aggravating uraemic pruritus and, to a lesser extent, directly, but in a way that is not related to the intensity of the xerotic lesions. Younger age is also an aggravating factor. More careful therapeutic management of uraemic xerosis and pruritus is needed to improve the QoL of patients with MRD. This includes, in particular, the use of a suitable emollient therapy as a primary measure.
ACKNOWLEDGEMENTS
The authors acknowledge the contribution of the members of the Uraemic Xerosis Working Group: Peter Arenberger, Mauro Barbareschi, Didier Bessis, Ligia Brzezinska-Wcislo, Bozena Chodynicka, Amedeo F De Vecchi, Alexandre Dumoulin, Peter Elsner, Corinne Ghienne, Wieslaw Glinski, Dimitrios Ioannides, Lajos Kemeny, François Maurice, Thomas Mettang, Claudio Ponticelli, Tomasz Szepietowski, Lukasz Matusiak, Georgia Orfanaki and Uwe Wollina.
The authors would also like to thank Nora Rahhali for her contribution to the statistical analysis, Isabelle Jeu for the preparation of the manuscript, and John Pimm for English language revision of the manuscript.
Conflict of interest: This study was financially supported by the Orfagen Company, of which A. Taberly is the statistician consultant and P. Dupuy an employee. The other authors declare no conflict of interest in the study, apart from having been financially supported by Orfagen for their participation in the therapeutic trials.
REFERENCES