Benjamin Bernuz, MD1,2, Haudrey Assier, MD3, Helene Bisseriex, MD2,
Jean-Baptiste Thiebaut, MD4, Celia Rech, MD2 and Alexis Schnitzler, MD2
From the 1Neurorehabilitation Unit, Leon Berard Hospital, Hyeres, 2Department of Physical Medicine and Rehabilitation, Raymond Poincare Hospital, Versailles University, Garches, 3Department of Dermatology, Henri Mondor Hospital, Paris XII University, Creteil and 4Department of Neurosurgery, Rotschild Foundation, Paris, France
CASE REPORT: A 43-year-old woman with cerebral palsy and disabling spasticity underwent a series of 4 implantations of intrathecal baclofen pumps, performed by two teams. A history of 3 aseptic local skin reactions over the site of insertion started 4 months after the first insertion, once with partial pump exposure. There were no clinical or biological signs of infection. Skin patch tests were negative. Relocation of the system was followed by a relapse, while removal of the pump was followed each time by complete resolution of the symptoms. Histological findings showed slight mononuclear dermal infiltration without epidermal lesions, which excluded contact dermatitis. Pump intolerance with a foreign-body reaction was diagnosed. A pump wrapped with polyethylene terephthalate was reimplanted. No recurrence of symptoms occurred after a 3-year follow-up period, with improvement in impairment, activity and satisfaction due to intrathecal baclofen therapy.
CONCLUSION: A foreign-body reaction after intrathecal baclofen pump implantation is a rare complication, which has not been reported previously, and which is associated with negative skin patch tests. In cases with no signs of infection, skin intolerance must be suspected and dermatological assessments should be carried out. Replacement with a pump wrapped in an inert coating is an effective and available solution.
Key words: baclofen pump; cerebral palsy; dermatitis; foreign-body reaction; spasticity.
J Rehabil Med 2012; 00: 00–00
Correspondence address: Benjamin Bernuz, Hopital Leon Berard, Avenue du Dr Armanet, BP 10121, FR-83418 Hyeres cedex, France. E-mail: b.bernuz@leonberard.com
Submitted July 5, 2011; accepted October 21, 2011
Introduction
Contact sensitivity and/or foreign-body reactions are rare and unrecognized complications following insertion of an intrathecal baclofen (ITB) pump. Differential diagnosis from skin infection can delay a positive diagnosis. Knowledge of, and the ability to identify, this phenomenon is therefore important. We report here a case of a patient who developed a series of 3 aseptic skin reactions following ITB insertion.
Case report
A 43-year-old woman with cerebral palsy, with no history of allergy or contact dermatitis, who had a disabling spasticity was implanted with an ITB pump (Synchromed II, Medtronic, USA) on the right lumbar wall after a positive ITB test showing improvement in gait parameters and lying and sitting discomfort. Four months later she developed painful erythema around the pump, with adherence and partial pump exposure, away from the scar. Her temperature, serum white blood cell count and fraction (neutrophil count 3.89 × 109/l; lymphocyte count 1.94 × 109/l; eosinophil count 0.08 × 109/l; monocyte count 0.83 × 109/l, basophil count 0.05 × 109/l), C-reactive protein (CRP) count (< 5 mg/l), and blood/skin cultures were normal.
The reaction was thought possibly to be due to the mechanical effect of friction from the wheelchair because of the pump position; thus, the pump was relocated to the right lower portion of the abdominal wall. One month later, the same skin reaction happened over the scar, still with no clinical or biological signs of infection. Topical corticosteroids and vitamin A were applied “successfully” for 10 days, after an infection symptom-free interval of 1 month, leading 1 week later to a real scar infection with fever (38ºC), CRP = 19 then > 50 mg/l, neutrophil count 9.6 × 109/l, and the presence of Staphylococcus aureus on skin culture. The pump was removed and antibiotics were administered with good results.
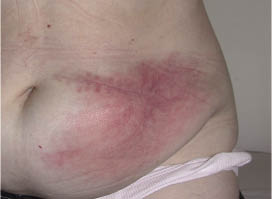
Six months later, another pump was implanted on the other side of the abdominal wall. This was followed by pruritic erythema of the scar after a two-month period (Fig.1), still with no signs of infection except for a slightly raised CRP count (13 mg/l), which was in a steady state. Skin patch tests were performed twice, first on the back, then on the arm, using a component sample set obtained from the manufacturer. The sample set included titanium, parylene-coated titanium and platinum iridium (metal), polyurethanes, silicone rubbers, and polysulfones (header and lead). Patch tests with titanium powder (titanium dioxide 1% petroleum) and with baclofen were also performed. The patch test results were interpreted according to the International Contact Dermatitis Group criteria. None of the patch tests were positive after 48 h (on the back) or 96 h (on the arm). Finally, tests were carried out with the baclofen-filled pump itself and its catheter; these tests were also negative.
Fig. 1. Final pruritic erythema prior to implantation of a polyethylene terephthalate-wrapped pump.
Histological findings from a skin biopsy showed a slight mononuclear dermal infiltration, mostly perivascular, without epidermal lesions, which excluded contact dermatitis.
The pump was finally removed and replaced with a polyethylene terephthalate (PET)-wrapped pump, which was approved and provided by the manufacturer. No recurrence of the skin reaction was observed during a follow-up period of 3 years. (A “time-line” of events is shown in Fig. 2.)
| 1st imp. 2005/11 | Relocation 2006/04 | T. CorticoS. 2006/06 | Removal 2nd imp. 2006/07 2007/02 | Removal 2nd imp. + insertion 3rd imp. 2008/02 |
| |
| Erythema 2006/03 | Erythema 2006/05 | Scar infection 2006/07 | Erythema 2007/04 |
Fig. 2. Time-line for medical procedures and skin reactions. imp.: implantation; T. CorticoS: topical corticosteroids.
Discussion
The first description of skin intolerance after implantation of a subcutaneous system was a case report of pacemaker contact dermatitis, published in 1970 (1). Such cutaneous complications have also been reported a few times for some other systems, such as spinal cord stimulators (2). To our knowledge, no report of ITB pump intolerance has been published, even though some components are shared with the other implantable systems, and its use in patients with cerebral palsy or spinal cord injury has been generalized because of its good cost-effectiveness ratio (3).
The primary diagnosis to exclude is an infection over the surgical site, which often leads to delayed recognition of skin intolerance, partly because of the lack of credit given to such a diagnosis. In this case report, many confounding factors applied: two surgical and rehabilitation teams were involved, there was initial delayed recognition until the second skin reaction, a scar infection occurred because of the triggered immune reaction after the corticosteroid treatment, and skin patch tests were negative after the third insertion.
Clinical features of skin intolerance include erythema over the insertion site, but generalized dermatitis (4) or isolated distant reactions have also been described (5). The time to the development of symptoms is widely variable: from 2 days to 2 years. The absence of fever and normal biological and culture results must lead to skin patch tests, even if subacute infections with normal biological results are reported (6). Although patch tests do not have 100% sensitivity, a negative result suggests another mechanism than contact dermatitis with delayed hypersensitivity (type III or IV), which remains the most common reaction (7, 8). A foreign-body reaction can occur (2), as for transplant rejection, and was retained in this case because of the biopsy result associated with negative patch tests. However, this discussion about physiopathology does not alter the decision to remove the pump, which remained as the only definitive treatment.
The successful use of polytetrafluoroethylene as an “inert” coating has been reported in cases of pacemaker dermatitis (9) and appears to be a real alternative to the definitive removal of implanted systems. PET is another inert coating, which produced excellent results in this case of foreign-body reaction, and which is available from the pump manufacturer.
In conclusion, a foreign-body reaction following implantation of an ITB pump is a rare complication that has not been reported previously, and which is associated with negative skin patch tests. In cases with no signs of infection, skin intolerance must be suspected and dermatological assessments should be carried out. Replacement of the device with a pump wrapped in an inert coating is an effective and available solution.
Acknowledgements. The authors acknowledges the whole MPR Department team of Garches Hospital, especially the Widal 1 Unit, in memory of La compagnie des lapins bleus.
References