Oliver Kaut, MD1, Benjamin Becker, PhD2, Christine Schneider, MD3, Feng Zhou, MSc2, Klaus Fliessbach, MD, PhD3,4, René Hurlemann, MD, PhD5 and Ullrich Wüllner, MD, PhD1,3
From the 1Department of Neurology, University of Bonn, Bonn, Germany, 2Center for Information in Medicine, Key Laboratory for NeuroInformation, University of Electronic Science and Technology of China, Chengdu, China, 3German Center for Neurodegenerative Diseases (DZNE), 4Department of Psychiatry, University of Bonn, and 5Division of
Medical Psychology, University of Bonn Bonn, Germany
OBJECTIVE: Whole-body vibration can be used to supplement canonical physical treatment. It is performed while probands stand on a vibrating platform. Therapeutic vibration can be generated as a stochastic vibratory pattern, referred to as stochastic resonance whole-body vibration (SR-WBV). Despite the widespread use of SR-WBV its neurophysiological mechanism is unclear.
DESIGN: A randomized sham-controlled double-blinded trial was performed as a pilot study. The experimental group received 6 cycles of SR-WBV at a frequency of 7 Hz with the SR-Zeptor device, and the sham group received the same treatment at a frequency of 1 Hz. At baseline 1.5 T functional magnetic resonance imaging (fMRI) was performed in the resting state, together with a finger/foot tapping test. A second fMRI was carried out after SR-WBV as sham treatment in both groups. Subsequently, a second cycle of SR-WBV was performed as sham or verum with consecutive fMRI, followed by a final fMRI on day 2.
SUBJECTS: Nineteen healthy volunteers were allocated to the experimental or sham group, respectively.
RESULTS AND CONCLUSION: Analyses of specific effects revealed a significant treatment × time interaction effect (p < 0.05, small-volume corrected (SVC FWE-corrected)) in the left caudate nucleus during intermediate difficulty when comparing pre- vs post-SR-WBV treatment in the verum group. This proof-of-concept study suggests the existence of cerebral effects of SR-WBV.
Key words: physical and rehabilitation medicine; vibration; magnetic resonance imaging.
J Rehabil Med 2016; 00: 00–00
Correspondence address: Oliver Kaut, Department of Neurology, University of Bonn, 53105 Bonn, Germany. E-mail: oliver.kaut@ukb.uni-bonn.de
Accepted Jul 7, 2016; Epub ahead of print Sep 26, 2016
INTRODUCTION
Biomechanical devices, including systems delivering whole-body vibration (WBV), have increasingly been used for the treatment of neurological movement disorders in addition to canonical physiotherapy in recent years. WBV is performed while patients stand on a vibrating platform. Therapeutic vibration can be generated either as non-stochastic (i.d. a sinusoidal, non-random) activity, or as stochastic (i.d. a non-sinusoidal, random) vibratory pattern (1). The latter is referred to as stochastic resonance therapy (SRT; resp. SR-WBV). In physics stochastic resonance (SR) is a non-linear cooperative effect in which a weak periodic stimulus entrains large-scale fluctuations resulting in enhancement of the weak component (2). There is also evidence for SR in living systems. A study using the mechanoceptor hair cells of the crayfish Procambarus clarkii demonstrated that weak signals can be enhanced by addition of an optimal level of external random signals in single sensory neurones (3).
Applying vibration therapy in humans in the 1990s, Schmidbleicher & Haas designed a mechanical device for musculoskeletal rehabilitation of downhill skiers, comprising independent oscillation platforms to stand on, using an unsynchronized multidimensional, low-frequency stimulus pattern superimposed on a sinusoidal basic activity (4). Positive effects were later observed in Parkinson’s disease (PD) (5). We recently confirmed an improvement in core motor symptoms, such as bradykinesia and postural stability in PD (6), and provided evidence of improved gait and speech in spinocerebellar ataxias (SCA; 1, 2, 3, 6) after treatment with stochastic resonance therapy (7).
Amelioration of clinical scores after non-SR WBV has also been shown in multiple sclerosis (8).
Despite initial reports of the effects of SR on isolated neuronal cells, to date it has remained unclear whether SR-WBV exerts stimulating effects in the human central nervous system (CNS), which might explain why SR-WBV is efficacious in complex neurological conditions, such as PD or multiple sclerosis.
This proof-of-concept study investigated the effects of SR-WBV in the brain using functional magnetic resonance imaging (fMRI) in young healthy individuals. The aim of the study was to test whether SR-WBV induces specific activation patterns in the brain. Given that the basal ganglia are the key neural substrates for motor function and have been abundantly involved in the pathology of PD, the analysis specifically focused on these nuclei (9).
METHODS
Study design
The 2-group design study was conducted in agreement with the principles of good clinical practice and the Declaration of Helsinki at the Department of Neurology, University of Bonn, Germany. The protocol was approved by the institutional ethics committee and all participants gave written informed consent (Lfd. no. 025/2012).
Healthy individuals were allocated either to the experimental or to the sham group using a block randomization with AABB distribution model (A = experimental; B = sham). All participants were blinded with regard to their assignment to the experimental or sham group. They were instructed to stand on the platform with eyes open, regular footwear and to adopt a semi-squat position with knees flexed slightly. A SR-Zeptor® with interference function “noise” (Human Mobility, Berlin, Germany) was used to perform SR-WBV.
The sham-condition was a treatment with the lowest frequency possible (level 1, amplitude 3 mm, no interference function, called “noise”) with SR-WBV consisting of 6 stimuli of 60 s duration; resting time between stimuli was 60 s. This treatment is known to have no effect on motor performance in patients with PD (5). The verum-condition consisted of a treatment with SR-WBV (level 7, amplitude 3 mm, including the interference function at level 3) with 6 stimuli of 60 s duration; resting time between stimuli 60 s.
Both the verum and the sham group first received a sham treatment with 2 fMRI sessions taking part immediately before (baseline) and after this session (post_1) in order to control for simple repetition effects which often impair serial fMRI experiments. Participants were then subjected to either sham or verum treatment according to their group allocation. A second post-treatment fMRI (post_2) was performed. A final follow-up fMRI (post_3) was performed 24 h later (Fig. 1A). Thus the experimental group was exposed to sham treatment first, then verum treatment (sham-verum), the sham group to a sham treatment, followed by a second sham treatment (sham-sham).
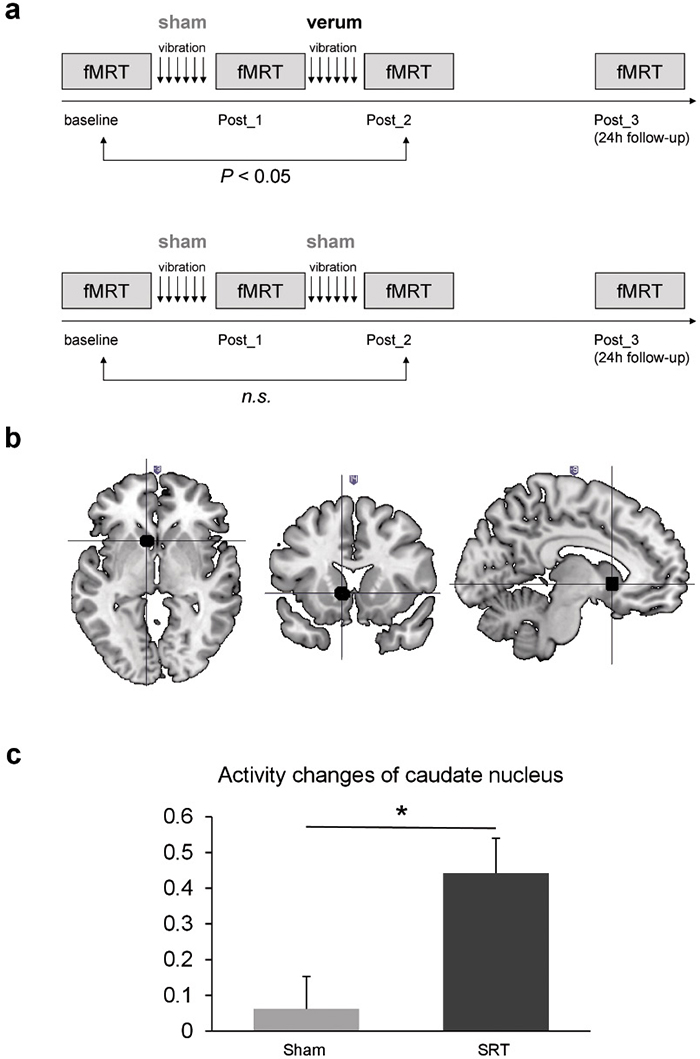
Fig. 1. (A) Study design of the sham group (upper fig.) and the verum group (lower fig.). (B) Signals (black) show increased activity of the left caudate nucleus after stochastic vibration therapy. (C) Activity of the left caudate nucleus during unilateral movement of the right hand and right food revealed no change in the sham group, but in the verum group increased activity in the caudate nucleus was detectable comparing pre- and post-treatment.
Subjects
Nineteen healthy individuals were investigated; 9 (6 females, 3 males; age mean 23.4 years (standard deviation (SD) 3.8); all right-handed; 1 left-handed individual was excluded resulting in 9 probands) were allocated in the experimental group (sham-verum), 10 (5 females, 5 males; age mean 24.4±3.7 years; all right-handed) in the sham group (sham-sham). Exclusion criteria included neurological diseases or relevant orthopaedic diseases in particular joint injuries.
Image acquisition
Scanning was performed on a 1.5 T Avanto Scanner (Siemens, Erlangen, Germany) using an 8-channel head coil. Functional data were acquired using echo-planar imaging (EPI)-sequences with a repetition time (TR) of 2.5 s, an echo time (TE) of 45 ms and a Flip angle of 90°. Thirty-one axial slices covered the whole brain including the superior part of the cerebellum and midbrain. Image resolution was 64 × 64 pixels with a field of view of 192 × 192 mm. Together with a slice thickness of 3 mm and an interslice gap of 0.3 mm, this resulted in a voxel size of 3 × 3 × 3.3 mm.
Task
During scanning subjects were asked to perform hand and/or foot movements (repeated alternating opening/closing of a fist and repeated alternating dorsal/palmar foot flexion). There were 8 movement conditions (left hand alone, left foot alone, left hand and left foot, left hand and right foot, right hand alone, right foot alone, right hand and right foot, right hand and left foot) and one rest condition (no movement). Each of these 9 conditions was repeated 8 times during one session. The order of the conditions was randomized with the constraint that the one condition was not repeated twice in a row. Subjects saw the active condition (indicated by the written words “left hand”, etc.) on a display presented by video goggles. They were instructed to repeat the movement for 10 s, during which the instruction was displayed. After a short pause with a fixation cross displayed (1,000–3,000 ms), the next condition started (Fig. 1A).
Pre-processing and analyses of functional imaging data
Functional MRI data was analysed with SPM8. Pre-processing of the EPI-images included slice time correction, motion correction, spatial normalization using des NewSegment algorithm implemented in SPM8 with the mean EPI-image as source image, and smoothing with a Gaussian kernel with full-width at half-maximum of 8 mm. Condition-specific regressors and movement parameters were modelled on the first level and convolved with the standard haemodynamic response function as provided in SPM. To increase the power to detect treatment-effects the conditions composite contrasts were computed according to task difficulty and associated engagement of the motor system. The low-difficulty contrast included the conditions that required individuals to move a single entity (e.g. left hand alone), the intermediate-difficulty contrast included conditions that required participants to move entities from the same body-side (e.g. right hand and right foot), finally the high-difficulty contrast incorporated conditions that required participants to move contralateral entities (e.g. right foot and left hand).
SPM repeated-measures analysis of variance (ANOVA), with the between-subject factor “treatment” (treatment vs sham) and the within-subject factor “time” (pre-treatment vs post-treatment), were employed as implemented in SPM’s flexible factorial module to examine non-specific treatment effects as well as specific treatment effects on the 3 levels of motor system engagement in terms of task difficulty. To account for the small sample size and a potential non-normal distribution of the data a non-parametric data analysis approach with 10,000 permutations using SnPM was used (http://www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/software/ snpm) (10).
Based on our a priori hypotheses that WBV stimulates the caudate/putamen the analyses focused on these regions using structural regions of interest from the Automated Anatomic Labellin atlas. Differences between the treatment sessions that exceeded a small-volume corrected (SVC) threshold of 0.05 corrected for multiple comparisons using family-wise error correction were regarded as significant. To further disentangle the observed neural effects individual parameter estimates were extracted from 6-mm radius spheres centred at the significant interaction effect to specifically examine changes between pre- and post-treatment within the treatment groups. The interaction effect was assessed using individual differences between pre- and post-treatment, then direct comparisons within the groups were computed to disentangle the direction of the effects. Again, non-parametric tests with a significance threshold of p < 0.05 were employed.
RESULTS
Subsequent non-parametric analyses of specific effects on the levels of task difficulty revealed a significant treatment × time interaction effect in the left caudate nucleus (located at –9/14/–2 in MNI space (from the Montreal Neurological Institute), p = 0.03, SVC-FEW-corrected, 10,000 permutations) during intermediate difficulty comparing pre- vs post-SRT treatment (Fig. 1B). Extraction of parameter estimates confirmed significant differences in caudal activity changes between the groups (p = 0.0124, 10,000 permutations). Direct comparison of pre- and post-treatment caudal activity within the groups revealed that the interaction was driven by significantly increased activity after SR-WBV (p = 0.002, 1.024 permutations, mean difference: 0.44, standard error of the mean (SEM) 0.09), in the absence of changes following sham treatment (p = 0.515, 1,024 permutations, mean difference: 0.06, SEM 0.09) (Fig. 1C).
No treatment effects were observed in the low-difficulty and bilateral high-difficulty conditions and in the 24 h follow-up.
DISCUSSION
The aim of this study was to test whether SR-WBV induces specific activation patterns in the brain, in particular the basal ganglia. fMRI revealed a distinct change in caudate nucleus activity in healthy controls after WBV. Importantly, changes were specifically observed after SR-WBV treatment, but not after sham treatment. To the authors’ knowledge, this is the first study to demonstrate an influence of stochastic resonance therapy on cerebral nuclei in the human brain.
The involvement of cerebral structures in the SR response has previously been suggested based on experiments using motor-evoked potentials from transcranial magnetic stimulation (TMS). Kossev et al. (11) reported that motor-evoked potentials of TMS were augmented when direct vibration was applied to the extensor carpi radialis muscle. However, since motor-evoked responses to TMS depend on cerebral, spinal and peripheral transmission these effect cannot unequivocally be attributed to the brain.
In rat brain WBV stimulates neurotransmitter expression (12), but this has never been replicated in a human study. Vibration signals activate the sensory receptors (muscle spindles), resulting in reflex muscle activation like the tonic vibration reflex (13). There is insufficient evidence to prove that this reflects the underlying mechanism of enhanced sensorimotor performance after WBV in a neurological disease like PD (14). We cannot disentangle whether the observed activation pattern is based on the vibratory stimulation at 7 Hz only or explicitly on the stochastic resonance input.
Our group and others have demonstrated the efficacy of SR-WBV in PD (5, 6). To date it remains unclear why SR-WBV leads to an amelioration of motor function in PD. An implication of improved muscle strength has been assumed. Improved muscle strength is correlated with gait velocity and movement velocity, and muscle weakness is one of the contributing factors to postural instability in PD (15). It has been shown that WBV leads to improvement in muscle strength (16), but this cannot explain why other motor symptoms of PD, such as tremor, are improved after vibration therapy. Thus, in diseases affecting the CNS, other or additional mechanisms of WBV-induced improvement have to be assumed. Our findings suggest that increased movement-related caudate nucleus activity contributes to SR-WBV effects. They fit well with the previous observation of others and our group, that a disease such as PD affecting basal ganglia including the caudate nucleus (17) responds positively to vibration therapy.
We found no vibration-associated effects in the bilateral high-difficulty task, but positive effects after the unilateral moderate-difficulty task. This lack of activation might be due to the fact that simultaneous movement of one limb on the right side and one limb on the left side recruits less-focused neuronal activity in the brain than the movement of 2 limbs on the same side. Single verum treatment with SR-WBV gained no lasting effects after 24 h. However, in PD long-term effects were detectable even after weeks when the treatment was performed several times (18). This suggests an accumulative impact of repeated SR-WBV treatment.
Study limitations
This study has some limitations. We did not correlate the cerebral activation with functional assessment of motor functions, such as faster walking or improved postural stability, in this cohort. However, we and others demonstrated this previously in patients with PD. Designed as a pilot study the number of subjects is small and the statistical power is limited. The reported findings therefore need to be regarded as preliminary and need further evaluation in larger samples. Regarding the neural effects of SRT in clinical populations, additional studies enrolling patients with PD are needed that assess treatment effects by means of fMRI to further evaluate associations between neural effects and clinical symptom-changes during the course of SR-WBV treatment.
In conclusion, the results of this proof-of-concept study provide initial insights into potential neural mechanisms of action of WBV. In particular, our results suggest the existence of cerebral effects of SR-WBV.
ACKNOWLEDGEMENTS
This work was supported by the University Clinic of Bonn, Germany and the Deutsche Parkinson Vereinigung (dPV). We thank all individuals involved for their participation.
The authors declare no conflicts of interest.
REFERENCES
