H. Jorn Bovenschen, Wil-Janne Gerritsen, Elke M.G. J. de Jong and Peter C. M. van de Kerkhof
Department of Dermatology, Radboud University Nijmegen Medical Centre, René Descartesdreef 1, 6525 GL, Nijmegen, The Netherlands. E-mail: j.bovenschen@derma.umcn.nl
Accepted December 20, 2005
Sir,
Alefacept (Amevive; Biogen Idec, Cambridge, MA, USA) was the first biological agent to be approved in the USA for moderate to severe chronic plaque psoriasis (1). It is a fully human fusion protein, consisting of the first extracellular domain of lymphocyte function- associated (LFA)-3 protein fused to the hinge, CH2 and CH3 domains of human IgG1 (1, 2). Normally, LFA-3 on antigen-presenting cells and CD2 on T cells interact to provide a co-stimulatory signal that plays a major role in T-cell activation (2, 3). Alefacept blocks this interaction (1, 4). Furthermore, natural killer cells and macrophages recognize the Fc portion of the alefacept molecule that has bound CD2+ T cells, leading to apoptosis of mainly activated CD2+ and CD45RO+ T cells, key players in psoriasis (2).
Efficacy and safety of an intramuscular alefacept course of 12 weeks have been shown in at least two phase III studies. The PASI-75 and PASI-50, well-recognized endpoints, were achieved by 28–33% and 53–75% of patients in these studies, respectively (5, 6). Comparable results have been presented for patients with more severe psoriasis in which conventional therapies had already failed (7). A limitation of alefacept therapy for psoriasis is a delayed maximal therapeutic effect, often reached only after the active 12-week course (8). With a potent topical corticosteroid a good clinical result is generally achieved prior to 4 weeks of treatment (9–12). However, long-term efficacy of topical corticosteroids is limited by tachyphylaxis and rebound following discontinuation of treatment. We studied the effect of a combination of these two treatments for psoriasis.
METHODS
The present prospective, randomized, double-blind, placebo-controlled, single-centre, explorative study included 16 patients with plaque psoriasis and compared the safety and efficacy of a 12-week 15 mg intramuscular alefacept course vs. a combination of alefacept and topical betamethasone dipropionate cream (Diprosone®, Schering-Plough Amstelveen, The Netherlands), once daily, in the first 4 weeks of treatment. All patients received alefacept, 8 were treated with betamethasone dipropionate cream, 8 with the vehicle cream alone (DiproBase®, Schering-Plough), once daily, maximal 50 mg per week. Both groups underwent a 12-week follow-up period. Topical treatment alone had proven insufficient in our patient group. Patients had been treated with at least one systemic anti-psoriatic therapy in the past, which had either failed or was contraindicated at the time of inclusion. Further criteria were similar to standard clinical trials to assess efficacy and safety of biological agents. Endpoints were safety and efficacy, determined by the following monthly assessed parameters: Psoriasis Area and Severity Index (PASI), quality of life (QoL), and self-reported psoriasis severity, determined using visual analogue scales (VAS), in the range 0–100, standard blood analysis, CD4+ T-cell count in peripheral blood and number and type of adverse events. Finally, the data were analysed with repeated measurement analyses of variance (ANOVA; Statistica®), where statistical significance was set at p < 0.05.
RESULTS
Although the treatment regimens did not show statistically significant differences, both showed a time-related statistically significant improvement in PASI (p < 0.01) (Fig. 1). The maximal mean PASI reduction was 58% in the combination group vs. 84% in the monotherapy group (p > 0.05). At week 4 and 8, none of the patients achieved PASI-50 in the combination group, vs. one patient (13%) in the monotherapy group. At week 12, in the combination group, 2 patients (25%) achieved PASI-50, but none of them reached PASI-75. This result was sustained until 3 months after the last injection. In contrast, in the monotherapy group, already after 4 and 8 weeks one patient (13%) had reached PASI-50. At week 12, 3 patients (38%) achieved PASI-50 and one of them reached PASI-75 at week 12. In this group even better results were achieved during 3 months of follow-up: 4 patients (50%) achieved PASI-50 and 3 of them reached PASI-75. Self-assessed psoriasis severity showed similar improvements, with a non-significant difference between the treatments at week 12 and beyond. The results were slightly in favour of the monotherapy group. The subjective scores correlated fairly well to the PASI (r = 0.41; p < 0.001). QoL results also showed a non-significant difference between the treatments at week 12 and during the follow-up phase. With both treatments QoL improved, again slightly in favour of alefacept monotherapy. The QoL scores correlated very well to the severity scores in each patient at the given time points (r = –0.32; p < 0.01), and to a lesser extent to the PASI (r = –0.09; p > 0.05)
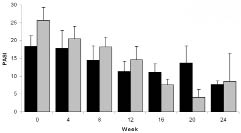
Fig. 1. PASI score (Mean + SEM) during and after 12 weeks treatment with alefacept in combination with 4 weeks of betamethasone dipropionate cream (black columns) or the vehicle cream (grey columns).
Adverse events were mild and similar to those observed in phase III and IV studies. No patients were withdrawn due to adverse events. In particular, infections were mild (e.g. common cold, viral laryngitis) and no doses were missed due to this. No clinically significant changes were observed in vital signs, physical examination and standard blood analyses. Peripheral blood T-cell counts were generally reduced from baseline. Only in the combination group, one dose of alefacept was missed as a result of a reduction in CD4+ T-cell count below the limit.
DISCUSSION
A statistically significant reduction in PASI scores was observed in both groups, treated with alefacept. Remarkably, the monotherapy group had more early responders, as well as long-term responders, than the combination group, although not statistically significant. This effect was empowered by a larger number of patients in the monotherapy group that had reduction of their self-reported psoriasis severity, as well as improvement of their QoL. Both parameters correlated relatively well to disease severity.
These results confirm the results of previous studies, regarding the clinical efficacy, effects on QoL and safety of alefacept monotherapy (5–8, 13–15). Potent topical corticosteroids have a broad range of effects on psoriatic plaques and most studies indicate a beneficial effect (9–12). Resistance to betamethasone dipropionate might be explained by tachyphylaxis, especially in our patient group, which had a long history of psoriasis and anti-psoriatic treatments including topical corticosteroids (12). Relapse after cessation of topical corticosteroid is a second factor that may in part account for the results (10, 11). Increasing the dose of the topical corticosteroid, prolonged topical treatment or co-treatment with other anti-psoriatic medications are further options to increase the efficacy of alefacept therapy (12).
In literature, and in the present study, there are clear-cut inter-individual differences in response to alefacept therapy (5, 6). For the future, it might be a challenge to identify predicting factors for response to biological agents in individual patients, prior to the start of treatment.
In conclusion, a combination of alefacept with a corticosteroid cream for 4 weeks, once daily, did not increase clinical efficacy and health-related QoL during a 12-week course of intramuscular alefacept and beyond, as judged by both physician and patient.
References
1. Ortonne JP, Prinz JC. Alefacept: a novel and selective biologic agent for the treatment of chronic plaque psoriasis. Eur J Dermatol 2004; 14: 41–45.
2. Majeau GR, Meier W, Jimmo B, Kioussis D, Hochman PS. Mechanism of lymphocyte function-associated molecule 3-Ig fusion proteins inhibition of T-cell responses: structure/function analysis in vitro and in human CD2 transgenic mice. J Immunol 1994; 152: 2753–2767.
3. Miller GT, Hochman PS, Meier W, Tizard R, Bixler SA, Rosa MD, Wallner BP. Specific interaction of lymphocyte function-associated antigen 3 with CD2 can inhibit T-cell responses. J Exp Med 1993; 178: 211–222.
4. Ellis CN, Krueger GG; Alefacept Clinical Study Group. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med 2001; 345: 248–255.
5. Krueger GG, Papp KA, Stough DB, Loven KH, Gulliver WP, Ellis CN; Alefacept Clinical Study Group. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol 2002; 47: 821–833.
6. Lebwohl M, Cristophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE; Alefacept Clinical Study Group. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003; 139: 719–727.
7. Van de Kerkhof PC, Griffiths CE, Cristophers E, Lebwohl M, Kruger GG. Alefacept in the treatment for psoriasis in patients for whom conventional therapies are inadequate. Dermatology 2005; 211(3): 256–263.
8. Krueger GG, Callis KP. Development and use of alefacept to treat psoriasis. J Am Acad Dermatol 2003; 49: 87–97.
9. De Jong EM, Ferrier CM, de Zwart A, Wauben-Penris PJ, Korstanje C, van de Kerkhof PC. Effects of topical treatment with budesonide on parameters for epidermal proliferation, keratinization and inflammation in psoriasis. J Dermatol Sci 1995; 9: 185–194.
10. Bovenschen HJ, Vissers WH, Seyger MM, van de Kerkhof PC. Selective persistence of dermal CD8+ T cells in lesional plaque psoriasis after clobetasol-17 propionate treatment. Acta Derm Venereol 2005; 85: 113–117.
11. Van der Vleuten CJ, van Vlijmen-Willems IM, de Jong EM, van de Kerkhof PC. Clobetasol-17 propionate lotion under hydrocolloid dressing (Duoderm ET) once weekly vs. unoccluded clobetasol-17-propionate ointment twice daily in psoriasis: an immunohistochemical study on remission and relapse. Arch Dermatol Res 1999; 291; 390–395.
12. Singh S, Reddy DC, Pandey SS. Topical therapy for psoriasis with the use of augmented betamethasone and calcipotriene on alternate weeks. J Am Acad Dermatol 2000; 43: 61–65.
