Adele Chedraoui1, Imad Uthman2, Ossama Abbas1 and Samer Ghosn1
Departments of 1Dermatology and 2Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Adele Chedraoui1, Imad Uthman2, Ossama Abbas1 and Samer Ghosn1
Departments of 1Dermatology and 2Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Adrenergic urticaria, a rare but distinct subtype of the physical urticarias, is characterized by wheals that are typically surrounded by a white halo of vasoconstriction, and by a positive response to intradermal adrenaline and noradrenaline injections. The pathogenesis of adrenergic urticaria is unknown. We report here a case of a 64-year-old woman with adrenergic urticaria who was found to have high levels of anti-double-stranded DNA antibodies without features of systemic lupus erythematosus. This is the first report associating adrenergic urticaria with anti-double-stranded DNA antibodies. The significance of this association is unknown. Key words: adrenergic urticaria; anti-double-stranded DNA antibodies.
(Accepted December 14, 2007.)
Acta Derm Venereol 2008; 88: 263–266.
Samer Ghosn, Department of Dermatology, American University of Beirut Medical Center, Riad El Solh St, PO Box 11-0236, Beirut, Lebanon. E-mail: sg03@aub.edu.lb
Adrenergic urticaria (AU), a rare but distinct subtype of the physical urticarias, is characterized by wheals that are typically surrounded by a white halo of vasoconstriction, and by a positive response to intradermal adrenaline and noradrenaline injections. The pathogenesis of AU is unknown. We report here a case of a 64-year-old woman with AU who was found to have high levels of anti-double-stranded DNA antibodies without features of systemic lupus erythematosus. This is the first report associating AU with anti-double-stranded DNA antibodies. The significance of this association is unknown.
Case report
A 64-year-old white woman was referred to the rheumatology department for evaluation of an elevated erythrocyte sedimentation rate (ESR). She reported a one-year history of recurrent episodes of an itchy evanescent rash over the distal extremities including the palms and soles. The rash was associated with burning sensation and pruritus over the palms and soles, lasting for minutes and disappearing spontaneously. The patient reported daily attacks, occurring mainly in the afternoon, following emotional stress and the intake of food items such as coffee, spices, ginger and aubergine. The episodes were not associated with palpitation, dyspnoea, syncope, tachypnoea, or perioral tingling, and they responded promptly but partially to antihistamine therapy. Recurrence shortly upon discontinuation of the antihistamine was the rule. The patient denied photosensitivity, oral ulcers, arthralgias and symptoms of serositis, neurological diseases or anxiety. She is known to be hypertensive and diabetic, taking bisoprolol, metformin, insulin and gliclazide. There was no family history of similar conditions or significant illnesses.
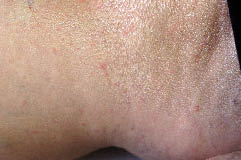
Physical examination revealed numerous few millimetre urticarial papules, each surrounded by a pale halo, over the upper and lower extremities (Fig. 1). There was no malar rash or other mucocutaneous lesions. The characteristic lesion could be reproduced locally by intradermal injection of either 10 ng noradrenaline or 10 ng adrenaline in 0.02 ml saline. Higher doses of adrenaline resulted only in blanching. An acetylcholine intradermal skin test was negative.
Fig 1. Numerous urticarial papules a few millimetres in diameter with a surrounding white halo over the right ankle.
Apart from the elevated ESR (45 mm/h, normal 0.0–2.5 mg/l), laboratory tests showed the following: C-reactive protein 6.1 mg/l (normal < 2.5 mg/l), haemoglobin 12.0 g/dl and haematocrit 36.0%. Antinuclear antibodies (ANA) test revealed positive fluorescence at 1:160 dilution (using HEp-2 cells). Anti-double-stranded DNA (anti-ds DNA) antibodies, measured twice by controlled enzyme-linked immunosorbent assay (ELISA), revealed high levels (530 IU/ml and 534 IU/ml, respectively, normal titre < 100 IU/ml). Lupus anticoagulant test was positive. Anti-Sm, anti-SSA, anti-SSB and anti-cardiolipin antibodies were undetected by ELISA. The other laboratory studies, including blood urea nitrogen, creatinine, urinalysis, 24-h urine protein, urine microalbumin, serum lipid profile, haemoglobin A1C, liver function tests and plasma IgE, were all within normal limits.
A biopsy taken from one urticarial papule revealed dilated lymphatic vessels in the papillary dermis and a sparse superficial perivascular mononuclear inflammatory cell infiltrate, consistent with an urticarial reaction. Based on all of these findings, the diagnosis of AU was made.
The rash was not under control despite the intake of bisoprolol for hypertension. Upon substitution of bisoprolol with propranolol (20 mg twice daily), complete resolution of the rash was achieved. A further increase to 20 mg 3 times daily was required to suppress the associated pruritus completely. The patient remained symptom-free over a follow-up period of 4 months. However, the rash recurred shortly after discontinuation of propranolol.
Discussion
As described by Shelley & Shelley (1) in 1985, AU is a rare but distinct form of stress-induced hives manifested as small erythematous papules surrounded by a white halo of vasoconstriction. Using an extensive PubMed search, we could retrieve only 5 cases of AU and one case of adrenergic pruritus (AP), the characteristics of which are shown in Table I. The lesions appear within 10–15 min after stress, coffee, chocolate or tea (1). In contrast to cholinergic urticaria lesions, the AU lesions are not induced by exercise or by an increase in body core temperature (1). The characteristic morphology is highly suggestive of the diagnosis, although the presence of a white halo surrounding urticarial lesions may be seen in arthropod bite reactions and in pruritic urticarial papules and plaques of pregnancy. These conditions could, however, be differentiated easily from AU on clinical grounds. The diagnosis of AU is confirmed by intradermal injection of 5 ng adrenaline or 3–10 ng noradrenaline, which will reproduce the characteristic lesions (2). The case described by Haustein (2) as adrenergic pruritus (case 4) speaks for a subset of AU presenting as pruritus without skin lesions. The fact that our patient required higher doses of propranolol to abort the residual pruritus may suggest that AP and AU represent opposite ends of the same disease entity, which will be referred to in this report as AU/AP.
Table I. Characteristics of all patients previously reported in the literature to have adrenergic urticaria (AU) or adrenergic pruritus (AP)
| Patient no. /diagnosis (Ref.) | Age (years)/sex | Triggering factors | Associated symptoms | Associated conditions | Intradermal test | Laboratory tests | Therapies used |
| 1/AU (1) | 28/M | Severe trauma (truck accident) Stress (after 10–15 min) Coffee Chocolate | Dyspnoea, Large urticarial wheals | Vitiligo | +ve with A and NA | Cat: high during attacks Hist, Dop and Ser: Nl IgE: high Cryoglobulins: –ve | Hyroxyzine 50 mg, and diphenhydramine 50 mg provided relief during attacks Propranolol 20 mg TID prevented attacks |
| 2/AU (1) | 40/F | Stress | None reported | None reported | +ve with NA (blanching with A) | Cat: high during attacks Hist, Dop, Prol and Ser: Nl. IgE: high Complement: Nl | Propranolol 10 mg QID decreased severity and frequency |
| 3/AU (2) | 51/M | Work stress Coffee (within 30 min) | None reported | Psycholabile | +ve with A and NA | Cat and Prol: high during attacks Hist and Ser: Nl IgE: Nl Cryoglobulins: –ve | Clemastine 2mg and ketotifen 2 mg provided relief during attacks Propranolol 25 mg TID and tolazoline suppressed attacks |
| 4/AP (2) | 34/F | Stress (within 1 h) | Pruritus | Psycholabile | Itching reproduced by A and NA | Cat and Prol : high during attacks Hist, Dop and Ser: Nl IgE: high | Propranolol 25 mg TID and tolazoline blocked itching |
| 5/AU (15) | 53/M | Stress of prick testing | Syncope | Reactive depression | +ve with A and NA | Not done | Propranolol 20 mg TID suppressed both urticaria and syncope |
| 6/AU (16) | 22/F | Stress (within 5–10 min) Exercise (without sweating) Winter | Pruritus | Easily frightened, anxious | +ve with A | Not done | Propranolol 20 mg and diazepam 2 mg prevented attacks |
M: male; F: female; A: adrenaline; NA: noradrenaline; Hist: histamine; Dop: dopamine; Ser: serotonin; Prol: prolactin; Cat: catecholamines; IgE: immunoglobulin E; Nl: within normal range; +ve: positive; –ve:negative; TID: 3 times a day; QID: 4 times a day.
The pathogenesis of AU/AP is not fully understood and many theories have been suggested. The autonomic dysfunction theory attributes the episodes of AU/AP to sympathetic hyperactivity based on the elevated plasma levels of noradrenaline, adrenaline and dopamine and the normal levels of histamine and serotonin often observed during the attacks (1). This may account for the other signs of dysautonomia (palpitations, paresthesias, tension, malaise, and tachypnoea) that may accompany the urticarial episodes. Often these attacks are triggered by stress or preceded by the intake of stimulants such as coffee and tea, all known to activate the adrenergic response. The fact that 4 out of the 7 AU/AP cases had some form of psycholability may also support the above theory, since abnormalities in the noradrenergic system are implicated in many anxiety and mood disorders (3). Sacerdote (4) described one case of urticarial episodes associated with hypoglycaemia in a man with insulin-dependant diabetes. These episodes rapidly disappeared upon ingestion of carbohydrates. This case further supports the hypothesis that sympathetic adrenergic discharge in response to hypoglycaemia may trigger urticarial episodes. This case, however, was not included in our review since the morphology of the lesions was not fully described in the report. In addition, Shelley & Shelley (1) described one patient with AU (case 1) who also had vitiligo. It has been speculated that the lower plasma catecholamine levels present in patients with vitiligo may upregulate their adrenergic receptors, leading to enhancement of the effect of sudden increases in adrenaline levels (5).
Another theory is the allergenic theory, which is supported by the usually elevated levels of IgE in 3 out of the 4 cases where it was assayed (Table I). Mast cells are known to have adrenergic as well as IgE receptors. It is not known whether the number of adrenergic receptors on mast cells is increased or the threshold of activation is lowered or whether noradrenaline acts synergistically with IgE on the mast cells in response to an unknown antigen, similar to what is seen in cholinergic urticaria (1). Mast cell degranulation and histamine release may be involved in the pathogenesis of AU/AP (1) accounting for the partial response to anti-histamine therapy in some cases (Table I). It is noteworthy that none of the AU/AP cases reported a personal or family history of atopy.
Our patient had normal IgE plasma levels and elevated anti-ds DNA antibodies. The significance of the latter finding remains unknown. Except for the coexistence of AU and vitiligo in one case, there are no reports of AU/AP occurring in the setting of autoimmune diseases. The patient, to the present time, does not fulfil the American College of Rheumatology (ACR) criteria to diagnose systemic lupus erythematosus (SLE) (6). Anti-ds DNA antibodies specificity for SLE approaches 97% (7). These antibodies have been reported to occur in healthy relatives of patients with SLE, and in association with infections, multiple myeloma, autoimmune hepatitis/cirrhosis (8) and the intake of certain drugs (9) such as penicillamine, isoniazid, methyldopa, TNF-α inhibitors, statins, minocycline and interferon-α. None of the medications used by our patient has been reported in association with the development of these autoantibodies. Haugbro et al. (10) suggested that only subpopulations of anti-ds DNA antibodies should be used in the diagnosis of SLE. The detection of these antibodies by ELISA has a positive predictive value of 95% for diagnosing SLE (10). These antibodies, however, have not been shown to play a pathogenic role in SLE (11). In addition, it should be noted that the appearance of the autoantibodies tends to follow a predictable course, with anti-ds DNA antibodies usually preceding the diagnosis of SLE by a mean of 2.2 years (12). Therefore, the possibility of SLE in our patient cannot be excluded and long-term follow-up is needed. SLE is not infrequently associated with urticarial reactions. Most of these reactions, however, fulfil the criteria of urticarial vasculitis and usually occur during the active stage of the disease (13). Only one report described solar urticaria as the presenting manifestation of SLE (14). Whether autoimmunity plays a role in at least a subset of AU/AP cases remains speculative, especially as none of the reported cases was investigated for autoantibodies.
AU/AP has been treated successfully with variable doses of propranolol that can be increased up to 40 mg 3 times daily (Table I). The response to propranolol can be used to confirm the diagnosis as well as to prevent attacks. Selective beta-1 adrenergic receptor blockers, such as atenolol (1) and bisoprolol (in our case), are usually not effective. How propranolol, a non-selective beta-adrenergic blocker, works in AU/AP is still unknown (5). The blockade of the beta-2 receptor on the mast cells might be implicated. Alternatively, a direct central nervous system effect of propranolol, which is known to cross the blood–brain barrier, remains a possibility. Tranquillizers or other agents/modalities that reduce autonomic discharge may provide relief, and antihistamines have been reported to have variable therapeutic responses (Table I).
The association of AU/AP, an extremely rare entity, with the development of anti-ds DNA antibodies deserves special mention. The role of these autoantibodies in the pathogenesis of AU/AP in the current case is difficult to determine. Further reports are needed to support a possible autoimmune basis for the pathogenesis of at least a subset of AU/AP.
ACKNOWLEDGEMENT
The authors would like to thank Merck-Medicapharm for their financial support. The current work was not affected in anyway by this financial grant.
References
1. Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress-induced hives. Lancet 1985; 2: 1031–1033.
2. Haustein UF. Adrenergic urticaria and adrenergic pruritus. Acta Derm Venereol 1990; 70: 82–84.
3. Anand A, Charney DS. Norepinephrine dysfunction in depression. J Clin Psychiatry 2000; 61 Suppl 10: 16–24.
4. Sacerdote A. Urticaria as a sign of hypoglycemia. Diabetes Care 1987; 10: 257.
5. Figueiredo A, Goncalo M, Paiva I, Poiares-Baptista A. Adrenergic urticaria. Diabetes Care 1988; 11: 440–441.
6. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277.
7. Habash-Bseiso DE, Yale SH, Glurich I, Goldberg JW. Serologic testing in connective tissue diseases. Clin Med Res 2005; 3: 190–193.
8. Isenberg D. Anti-dsDNA antibodies: still a useful criterion for patients with systemic lupus erythematosus? Lupus 2004; 13: 881–885.
9. Antonov D, Kazandjieva J, Etugov D, Gospodinov D, Tsankov N. Drug-induced lupus erythematosus. Clin Dermatol 2004; 22: 157–166.
10. Haugbro K, Nossent JC, Winkler T, Figenschau Y, Rekvig OP. Anti-dsDNA antibodies and disease classification in antinuclear antibody positive patients: the role of analytical diversity. Ann Rheum Dis 2004; 63: 386–394.
11. Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? Rheumatology 2007; 46: 1052–1056.
12. Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003; 349: 1526–1533.
13. Provost TT, Zone JJ, Synkowski D, Maddison PJ, Reichlin M. Unusual cutaneous manifestations of systemic lupus erythematosus: I. Urticaria-like lesions. Correlation with clinical and serological abnormalities. J Invest Dermatol 1980; 75: 495–499.
14. Delorme P, Giroux JM. Solar urticaria as the presenting manifestation of systemic lupus erythematosus. Can Med Assoc J 1966; 95: 539–542.
15. Maerens-Tchokokam B, Vigan M, Breuillard F, Vuitton DA, Girardin P, Laurent R. Guess what! Adrenergic urticaria. Eur J Dermatol 1999; 9: 137–138.
16. Vithayasai P, Vithayasai V. Adrenergic urticaria: a first report from Thailand. J Med Assoc Thai 1989; 72: 478–480.
