Rieko Kabashima1, Ryosuke Hino1, Toshinori Bito1, Kenji Kabashima1, Motonobu Nakamura1, Bungo Ohyama2, Takashi Hashimoto2 and Yoshiki Tokura1
Department of Dermatology, 1University of Occupational and Environmental Health and 2University of Kurume, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu, Fukuoka 807-8555, Japan. E-mail: r-kabaji@med.uoeh-u.ac.jp
Accepted December 3, 2009.
Epidermolysis bullosa acquisita (EBA) is a chronic subepidermal blistering disorder characterized by circulating IgG anti-basement membrane zone (BMZ) auto-antibodies (1, 2). The EBA antigen has been identified as type VII collagen (3). More than 100 patients with coexisting psoriasis and autoimmune bullous diseases, such as bullous pemphigoid (BP) and pemphigus vulgaris (PV), have been reported in the literature but there have been only a few such cases of EBA (3–5).
Case Report
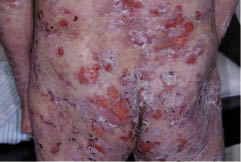
A 47-year-old Japanese man was referred to us for evaluation of a pruritic bullous eruption, which had appeared 3 weeks previously. He had been treated with oral cyclosporine (100–150 mg daily) for 10 years for psoriasis vulgaris. On examination, vesicles and flaccid blisters were present with an oedematous and erythematous base on his trunk and extremities (Fig. 1). No mucosal lesions were observed in the mouth, eyes or genitalia. On initial examination, there was no psoriasiform eruption.
Fig. 1. Vesicles and flaccid bullae with oedematous erythema on the trunk.
Laboratory data showed leukocytosis of 12,300/µl with 78.1% neutrophils and 2.0% eosinophils, elevated C-reactive protein (11.5 mg/dl; normal < 0.3 mg/dl), hypoalbuminaemia (1.7 g/dl; 4.0–5.0 g/dl), and anaemia (8.4 g/dl; 13.6–17.2 g/dl).
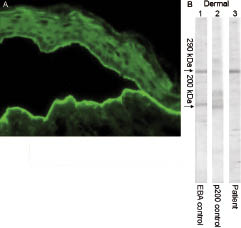
Skin biopsy specimens obtained from small vesicles revealed a subepidermal blister with infiltration of inflammatory cells composed largely of neutrophils and eosinophils. The histological signs of psoriasis, including parakeratosis, hyperkeratosis, and elongation of rete ridges, were absent in the specimen. Direct immunofluorescence (IF) showed a modest linear deposit of IgG and C3 at the BMZ. Indirect IF, using human skin non-treated or split with 1 mol/l NaCl (Fig. 2A) as a substrate, demonstrated IgG auto-antibodies that bound to the dermal side.
Immunoblot analysis was performed using ethylene-diaminetetra-acetic acid (EDTA)-separated normal human epidermal or dermal extracts, as described previously (6, 7). The patient’s serum reacted with 290 kDa of EBA antigen existing in the dermal extracts, but not with 200 kDa antigen of anti-p200 pemphigoid (Fig. 2B). When epidermal extracts were used, the patient’s serum showed no reactivity with 230 or 180 kDa of BP antigens, or 130 or 160 kDa of PV antigens.
Fig. 2. (A) Indirect immunofluorescence, showing IgG binding to the dermal side of human skin split with 1 mol/l NaCl. (B) Immunoblot analysis using ethylenediamine-tetra-acetic acid (EDTA)-separated normal human dermal extracts. Lane 1, positive control serum of epidermolysis bullosa acquisita (EBA); lane 2, positive control serum of anti-p200 pemphigoid; lane 3, our patient’s serum. The left-hand arrow indicates the position of the 290 kDa EBA antigen and the 200 kDa anti-p200 pemphigoid. Our patient’s serum yielded the same 290 kDa band as the control EBA serum, but did not show the 200 kDa band.
During hospitalization, oral cyclosporine (150 mg daily) was continued. Oral administration of prednisolone, 30–50 mg daily, was added. Because the bullous eruption was alleviated, prednisolone was tapered to 20 mg daily. Simultaneously, minocycline hydrochloride (100 mg daily) and nicotinic-acid amide (1200 mg daily) were added. However, the eruption was again exacerbated, with blister formation. The patient was treated with half-pulse methylprednisolone (500 mg daily for 3 days), followed by oral prednisolone, 40 mg daily. Dapsone (50 mg daily) was then commenced with prednisolone (30 mg daily). The combination of prednisolone and dapsone remarkably improved the erythema and blisters without formation of scars, but with milia. The patient is currently largely free from blisters with the continuing administration of oral dapsone (50 mg daily) and tapered prednisolone (5 mg daily).
Discussion
Although most patients with autoimmune bullous diseases associated with psoriasis have BP, anti-p200 pemphigoid, with tissue-bound and circulating autoantibodies to a 200 kDa protein, may be another major disease; in one study 19 of 61 patients with anti-p200 pemphigoid (31%) had associated psoriasis vulgaris (8). Treatments for psoriasis, such as ultraviolet (UV) B radiation and psoralen plus UVA (PUVA), may be implicated in the induction of bullous diseases, in particular BP (5). However, our patient had not been treated with phototherapy. It is possible that the long-term administration of cyclosporine masked the overt appearance of EBA.
Only 3 cases of coexistence of EBA and psoriasis have been reported in the literature (Table I). In cases 2 and 3, EBA was diagnosed by immunoblot analysis (3, 4). Endo et al. (4) reported a case whose serum reacted with the 290 kDa of EBA antigen in dermal extracts.
Table I. Reported cases of epidermolysis bullosa acquisita (EBA) associated with psoriasis
| Case (ref) | Age (years)/sex | Previous treatment for psoriasis vulgaris (duration) | Treatment for EBA |
| 1 (5) | 81/M | Tar + UVB (ND) | Methotrexate, 15 or 20 mg/week |
| 2 (4) | 37/M | Topical corticosteroids (20 years) | Etretinate, 75 mg/day + prednisolone, 60 mg/day |
| 3 (3) | 71/F | Topical vitamin D3 (2 years) | Prednisolone, 1 mg/kg/day + dapsone, 50 mg/day |
| 4 (this case) | 49/M | Systemic cyclosporine (15 years) | Prednisolone, 30 mg/day + dapsone, 50 mg/day |
ND: not described.
Nevertheless, there is a possibility that some cases reported as BP are in fact EBA or anti-p200 pemphigoid. For example, Kirtschig et al. (5) reported a psoriatic patient with a subepidermal blister disease, whose serum bound to a 250 kDa polypeptide.
Several lines of evidence have suggested that a numerical or functional reduction in regulatory T (Treg) cells specific for auto-antigens contributes to autoimmune bullous diseases. Treg cells specific for desmoglein 3 were detected less frequently in patients with PV (9). A case has been reported of pemphigoid nodularis, associated with immune dysregulation, polyendocrinopathy enteropathy, and X-linked syndrome caused by a mutation of the Foxp3 gene, which is indispensable for Treg-cell development (10). However, the role of Treg cells in EBM remains unclear. Depletion of the Treg cells with an antibody did not affect the autoimmune response type VII collagen in a mouse model (11). This mouse model reproduced auto-antibody production against type VII collagen, but not blistering (12). While psoriasis is an immunological disorder, with activated Th1 and Th17 cells triggering the inflammation and hyperproliferation of keratinocytes (13), Treg cells are also involved in the development of psoriasis. The percentage of dermal Foxp3-positive Treg cells in psoriatic patients was lower than in other inflammatory diseases (14). Moreover, adoptive transfer of wild type Treg cells into the mouse model of psoriasis, Cd18 hypomorphic PL/J mice, resulted in an improvement of psoriasiform eruptions (15). Thus, dysfunction and/or numerical reduction in Treg cells have been observed in both autoimmune bullous diseases and psoriasis, implying the association of EBA with psoriasis in our case.
When the standard treatments of EBA are unsatisfactory, alternative treatments are necessary and include colchicines, dapsone, photophoresis, infliximab and intravenous immunoglobulin. For the coexisting psoriasis, long-term administration of oral prednisolone is discouraged. Furthermore, our patient developed EBA despite being treated with 150 mg of cyclosporine daily. Both the conditions of EBA and psoriasis were eventually well controlled by oral dapsone (50 mg daily) and a low dose of prednisolone (5 mg daily).
References