Arnaud Duval1, Jacqueline Rivet2, Isabelle Moulonguet1, Olivier Cassar3, Félix Agbalika4, Daniel Wallach5, Antoine Gessain3 and Antoine Petit1
1Service de Dermatologie, 2Service d’Anatomie Pathologique, Hôpital Saint-Louis, 3Unité d’Epidémiologie et de Physiopathologie des Virus Oncogènes, CNRS URA 3015, Institut Pasteur, 4Service de Virologie, Hôpital Saint-Louis, Université Denis Diderot Paris 7 and 5Service de Dermatologie, Hôpital Cochin-Tarnier, Paris, France
Prurigo nodularis is a pruritic dermatosis of unknown origin. Human T-cell lymphotropic virus type 1 (HTLV-1) causes adult T-cell leukaemia/lymphoma. HTLV-1 is not considered to be a cause of prurigo nodularis. A 52-year-old black man, from the French West Indies, who had had prurigo nodularis for 12 years, presented with a distinct micropapular eruption with the typical pathological picture of epidermotropic T-cell lymphoma. Based on HTLV-1-positive serology and monoclonal integration of HTLV-1 we diagnosed smouldering adult T-cell leukaemia/lymphoma. Re-examinination of previous skin biopsies revealed that the disease had been evolving for 12 years. Treatment with α-interferon, 3 × 106 units three times a week, associated with zidovudine, 1 g daily, resulted in complete remission within 4 months. When investigating a prurigo nodularis, we therefore recommend: (i) performing HTLV-1 serology if the patient comes from an endemic area; (ii) if positive, performing CD25 staining and looking for a HTLV-1 clonal integration; and (iii) if positive, using a treatment targeting HTLV-1. Key words: HTLV-1; prurigo; human T-cell leukaemia-lymphoma; ATLL; pruritus; skin.
(Accepted December 17, 2009.)
Acta Derm Venereol 2010; 90: XX–XX.
Arnaud Duval, Service de Dermatologie, Hôpital Saint-Louis, 1, Avenue Claude Vellefaux, FR-75010 Paris, France. E-mail: arnaudduval@netcourrier.com
Prurigo nodularis (Hyde’s prurigo) is a chronic, pruritic, benign dermatosis of unknown origin. It is characterized by pruritic papules and nodules on the trunk and limb in areas accessible to scratching. The face is usually spared. Pathologically there is an acanthosis of the epidermis and a polymorphous inflammatory infiltrate in the dermis. Nerves are hypertrophic or increased in number. Clinical, biological and radiological investigations are necessary to rule out internal diseases, but human T-cell lymphotropic virus type 1 (HTLV-1) is not considered a cause of prurigo (1).
HTLV-1 is an enveloped, double-stranded RNA, type C (Retroviridae family, subfamily oncovirus) T-cell tropic virus. It causes a severe T-cell lymphoproliferation named adult T-cell leukaemia/lymphoma (ATLL), for which four types, based on clinical and biological criteria, have been described: acute, lymphoma, chronic, and smouldering. HTLV-1 infection has a much broader spectrum of disease manifestations, including HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), uveitis, and infective dermatitis mainly in children (2). The most common specific manifestation of ATLL seems to be a generalized maculopapular eruption. Prurigo nodularis is not considered a manifestation of ATLL (3).
We describe here a patient who had prurigo nodularis for 12 years before he developed an atypical eruption that led us to a diagnosis of smouldering ATLL due to HTLV-1.
CASE REPORT
A 52-year-old black man was referred to us for a nodular prurigo that had started 12 years previously. He had spent his childhood in Martinique (French West Indies). He had no significant medical history.
Numerous investigations were performed repeatedly during this period of time. Full blood count, creatininemia, calcemia, thyroid-stimulating hormone, and liver tests were normal. HIV, B and C hepatitis serologies were negative. Enzyme-linked immunosorbent assay (ELISA) HTLV-1 serology was known to be positive. No measures were taken at that time to treat HTLV-1. Thoracic and abdominal computed tomography (CT) were normal. Intestinal strongyloidosis was treated without any effect on the prurigo and rapidly relapsed. Repeated skin biopsies were consistent with prurigo. Topical very potent corticosteroids were ineffective. Thalidomide 100 mg daily was then initiated. After 3 months, the lesions disappeared and the treatment was interrupted. After several months pruritus and papules returned. Three years later, the patient presented a new flare, which was more severe, of nodular lesions on the face and ears.
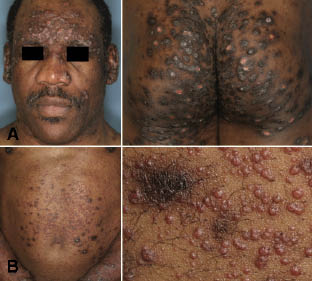
Upon admission, the patient was in good general condition, despite severe pruritus. Numerous, thick nodular lesions were present on the face, ears, limbs and, to a lesser extent, on the trunk. Lichenified plaques were present on the limbs (Fig. 1A). No lymph node enlargement or hepato/splenomegaly were found. Another biopsy was performed, consistent with lichenification. A 1% anthralin treatment associated with corticosteroid ointment (betamethasone dipropionate 0.05% 30 g, Vaseline 69 g)) was then initiated. After 10 days, the patient developed a new, acute, distinct micropapular eruption, especially on the trunk. Upon closer examination, papules were monomorphic. They were very superficial with an umbilicated centre, giving them the appearance of molluscum contagiosum (Fig. 1B).
Fig. 1. (A) Thick, excoriated, hyperpigmented nodules of prurigo on the face and buttocks before treatment. (B) Numerous umbilicated, flesh-coloured tiny papules appeared between hyperpigmented nodules and flattened residual lesions of prurigo after treatment with anthralin.
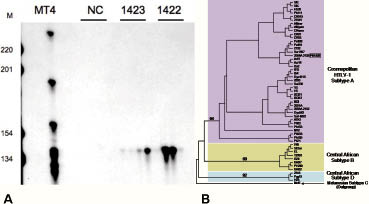
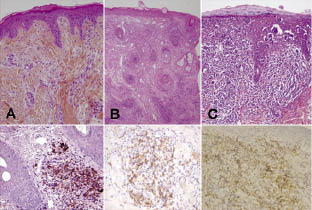
New investigations were performed. Blood cells detected in a blood smear comprised 45% flower cells (activated lymphocytes with convoluted nuclei and basophilic cytoplasm) and 99% of CD4+ lymphocytes were CD25 positive. A Western blot of the blood showed a typical complete seroreactivity (p19, p24, pr53 and GD21 and MTA-I). HTLV-1 antibody titre determined by serial dilution by immunofluorescence on MT2 cells was 1/2560. A high HTLV-1 viral load was found in the blood (135,993 cop/150,000 cells, log10 = 4.1) and in lesional skin. Micropapule biopsy revealed a high density dermal lymphocytic infiltrate with epidermotropism (Fig. 3C). The cells had atypical nuclei (flower cells). Lymphocytes were CD2+, CD3+, CD4+, CD5+, CD7–, CD8–, CD25+, CD20+, CD30–. CD25 positivity was consistent with activated T lymphocytes. Inverse polymerase chain reaction (PCR) demonstrated a clear monoclonal population both in peripheral blood mononuclear cells and in a cutaneous biopsy (Fig. 2A). Sequencing of a 522-bp fragment of the HTLV-1 env gene demonstrated that the virus infecting this patient belonged to the transcontinental subgroup of the Cosmopolitan subtype (Fig. 2B). Previous biopsies were read again (Fig. 3A and 3B). On the biopsy made 4 years previously, a significant lymphocytic infiltrate was found with nucleus abnormalities, but with no epidermotropism. On the biopsy made performed 12 years previously, the lymphocytic infiltrate was very sparse. However, on all biopsies CD25 staining was positive.
Fig. 2. (A) Ligation-mediated PCR analysis of human T-cell lymphotropic virus type 1 (HTLV-1) replication in peripheral blood mononuclear cells (PBMCs) DNA (1422) and skin biopsy DNA (1423) from the present case. For both samples, digested and circularized DNA was submitted to PCR analysis. Identical clones were detected four times in PBMCs and in skin lesions. M: molecular weight marker. NC: negative control; MT4 positive control. (B) Phylogenetic tree constructed by the neighbour-joining method performed in the PAUP program (v4.0b10) on a 522-bp fragment of the env gene for 55 HTLV-1 available sequences including the sequence generated in this work (underlined in bold type). A–D correspond to the four major HTLV-1 subtypes, and subtype A corresponds to the Cosmopolitan one including the PH1422 sequence generated in this work.
Fig. 3. (A) Biopsy of a prurigo lesion in 1997, Haematoxylin & Eosin (HE) staining × 200. Dermal infiltrate is polymorphous and sparse, but a few flower cells are present and CD25 staining is positive. (B) Biopsy of a nodule in 2004, HE × 200. Dense dermal lymphocytic infiltrate with nuclear atypia. CD25 staining is positive. (C) Biopsy of an umbilicated papule in 2008, HE × 200. Sub-epidermal dense lymphocytic infiltrate with significant epidermotropism (Pautrier’s abscess). CD25 staining is positive.
Based on these results, a diagnosis of the smouldering subtype of ATLL was made. The patient was treated with α-2a interferon, 3 × 106 units three times a week, and zidovudine, 1000 mg daily. After 4 months all the lesions and pruritus had disappeared. Biologically, CD25+ CD4+ lymphocytes returned to normal (33% of CD4+ lymphocytes). Six months later interferon was interrupted because of asthenia, and zidovudine was continued alone. One year later the patient was still in complete remission.
DISCUSSION
We describe here a case of a benign HTLV-1-related prurigo nodularis that turned into a smouldering ATLL, with an acute monomorphic peculiar eruption with pathological features of cutaneous T-cell lymphoma. Anthralin therapy may have triggered this new remarkable eruption, but it could be also a fortuitous event.
HTLV-1 has been implicated in prurigo nodularis only as a prodromal manifestation of ATLL (1, 4). In the case described here there is strong evidence of the implication of HTLV-1 in the initial prurigo, since we found CD25-positive lymphocytes in the first biopsy performed 12 years previously and since all the lesions disappeared with antiretroviral treatment. One question is how HTLV-1 could provoke prurigo. Several neurological diseases associated with HTLV-1 have been described in addition to typical HAM/TSP, such as polyneuropathy, but the causal role of the virus has not been established unequivocally. The pathophysiology is unknown and several hypotheses are proposed: direct toxicity, autoimmunity, and bystander damage (5). Hypertrophy of the nerves is a hallmark of prurigo nodularis (6) and polyneuropathy has been associated with nodular prurigo (7). Our patient presented the smouldering form of ATLL. This form is characterized by 5% or more abnormal lymphocytes of T-cell nature in the blood, a normal lymphocyte level, no hypercalcaemia, lactate dehydrogenase (LDH) value of up to 1.5 × the normal upper limit, no lymphadenopathy, no involvement of the liver, spleen, central nervous system (CNS), bone and gastrointestinal tract, and no ascites or pleural effusion. Skin and pulmonary lesion(s) may be present (8, 9). The diagnosis of ATLL is usually made on morphological analysis associated with a typical clinical context in a person originating from an endemic area (mainly the West Indies or Africa for patients treated in Europe). Indeed, cytological examination may reveal infiltration by ‘’cerebriform’’ or ‘’flower cells’’. It must be confirmed by clonal integration of HTLV-1 provirus in the host genome, either by classical Southern blot or inverse PCR, as in our case (10–12). Adult T -cell leukaemia or lymphoma develops after a very long latency period (30–60 years) in 3–5% of individuals infected with HTLV-1, and is preceded by oligoclonal expansions of activated T cells that have been infected with HTLV-1. HTLV-1 can be transmitted through sexual intercourse, or from mother to child through prolonged breastfeeding (2). Our patient came from Martinique, an area where HTLV-1 infection is endemic. We could not determine the form of transmission.
Cutaneous manifestations of ATLL are heterogeneous. They usually involve the entire body. Maculo-papular presentation is the most common. Papules, plaques, nodules, tumour, erythrodermia, and ichthyosis-like lesions, have been reported (3). To our knowledge anthralin-triggered eruption has not been reported previously. The presentation of ATLL described here is very unusual, since it began with a non-specific prurigo, developed into a prurigo nodularis-like eruption, with pathological features consistent with lymphoma seen after re-examination of a biopsy, and ended with a diffuse papular rash with a significant epidermotropism. Of note was the extension of the prurigo to the head, which is not commonly seen in prurigo nodularis (1). This presentation can be seen either as a continuum of ATLL manifestations or as an HTLV-1 infection associated with non-specific prurigo complicated later with ATLL. This is the third case reported of long-lasting prurigo preceding ATLL (4, 9).
Treatment of ATLL depends on the severity of the disease. Concerning smouldering forms, treatment with α-interferon and antiretroviral therapy, such as zidovudine, is recommended and can be used as induction and maintenance therapy (8).
Of note is the previous complete remission of the disease with the introduction of thalidomide, initiated to treat a refractory prurigo. Thalidomide is an immunomodulatory agent with demonstrated activity in multiple myeloma, mantle cell lymphoma and lymphoplasmacytic lymphoma and refractory prurigo nodularis. Despite few case reports there is a lack of evidence concerning its efficacy in non-Hodgkin’s lymphoma (13–15). Since the lymphocytes in HTLV-1-related lymphoma are activated one might wonder whether this type of lymphoma has a stronger propensity to respond to this therapy. There is no case reported of HTLV-1 lymphoma treated with thalidomide.
This case provides further evidence of a link between prurigo and HTLV-1 infection. We recommend that HTLV-1 serology be performed systematically when treating prurigo for patients who come from an endemic area. If the serology is positive, we recommend checking for flower cells in the blood smear and in the skin infiltrate, as well as CD25 staining and viral load in the plasma and skin. If these tests are positive, a strong argument can be made for giving specific anti-HTLV-1 treatment, especially if monoclonal integration of the virus is proven. This treatment may relieve the patient from a distressing dermatosis and prevent the HTLV-1-related prurigo from developing into a more aggressive HTLV-1 complication.
The authors declare no conflict of interest.
REFERENCES