Miklós Sárdy, Andreas Wollenberg, Andrea Niedermeier and Michael J. Flaig
Department of Dermatology and Allergology, Ludwig-Maximilian University, Munich, Germany
Miklós Sárdy, Andreas Wollenberg, Andrea Niedermeier and Michael J. Flaig
Department of Dermatology and Allergology, Ludwig-Maximilian University, Munich, Germany
Epstein-Barr virus (EBV) infection may rarely be associated with genital ulcers (ulcus vulvae acutum), a very painful manifestation. The aetiopathogenesis of the disease is not fully understood. We describe here a case of an adolescent virgin with multiple, deep genital ulcers associated with acute infectious mononucleosis. The diagnosis was supported by the clinical symptoms, atypical lymphocytosis, elevated circulating levels of liver enzymes, positive EBV serology, and the detection of EBV in a swab sample and a biopsy specimen by PCR. The virus could not be detected by immunohistochemistry or in situ hybridization. After a short course of methylprednisolone as a supportive treatment, the ulcers healed within one month. No relapse occurred during the 2-year follow-up. Available data relating to the aetiopathogenesis of this condition are reviewed, and we speculate that it may have been caused by percutaneous autoinoculation through cervicovaginal fluid. Key words: infectious mononucleosis; Epstein-Barr virus; human herpesvirus 4; acute genital ulcer; vulvar ulcers; Lipschütz’s ulcer; aphthosis.
(Accepted June 23, 2010)
Acta Derm Venereol 2011; 91: 55–59.
Miklós Sárdy, Department of Dermatology and Allergology, Ludwig-Maximilian University, Frauenlobstr. 9-11, DE-80337 Munich, Germany. E-mail: miklos.sardy@med.uni-muenchen.de
Epstein-Barr virus (EBV) is a lymphotropic, double-stranded DNA virus classified as the fourth member of the Herpesviridae family (human herpesvirus 4, HHV-4) (1). It is primarily transmitted through direct contact with saliva. Transmission via blood transfusion or genital contact is also possible. Aerosols or fomites are unlikely sources of infection. The infection is generally asymptomatic, but in a minority of patients it can manifest as infectious mononucleosis, which is associated with a variety of mucocutaneous and systemic presentations. In addition, latent, persistant infection with EBV has been implicated in a number of conditions, including Burkitt’s lymphoma, nasopharyngeal carcinoma and systemic lupus erythematosus (1–3). Ulcus vulvae acutum (also referred to as Lipschütz’s ulcer) is a clinically-defined ulcerative disease (4). It represents a very rare (and thus probably underdiagnosed) complication of acute, primary EBV infection. Only around 40 cases have been reported (for reviews, see (5, 6)). We describe here an otherwise healthy, adolescent female with an episode of ulcus vulvae acutum associated with infectious mononucleosis.
CASE REPORT
A previously healthy, post-menarcheal, 16-year-old female patient presented with a two-week history of a sore throat and a tender, stiff neck, and a one-week history of an itching genital lesion that progressed to multiple, very painful ulcers. Her sore throat and neck had been treated with diclofenac (75 mg twice daily) for 2 days before appearance of the genital symptoms. She noticed the ulcers 4 days before presentation, and treated them with local antiseptics. She was a virgin and denied any previous sexual activity and thus the possibility of genital herpes infection. Other associated symptoms included dysuria and symmetrical, generalized polyarthralgia of the extremities.
On physical examination, she had enlarged and erythematous tonsils, mild tenderness caused by anterior cervical lymphadenopathy, and a slightly enlarged liver, but no lymphadenopathy in any other region or splenomegaly. Ophthalmological evaluation was normal. Genital examination revealed a large, well-circumscribed, deep ulcer of 4.5 cm diameter with vivid purple edges and covered with a thick fibrinous membrane on the medial surface of the right labium minus, as well as multiple, smaller and shallower ulcers on both labia majora, extending to the proximal and medial parts of upper thighs (Fig. 1). In addition, the patient had a greyish vaginal discharge.
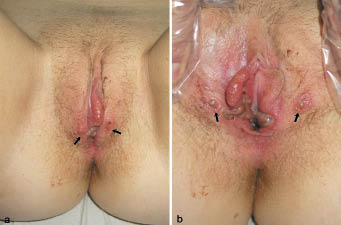
Fig. 1. Clinical appearance of the genital region on day 4. (a) Multiple, herpetiform oval or round, focally coalescent, partially symmetrical (“kissing”, arrows), superficial ulcers on the labia, covered by fibrinous membranes and surrounded by vivid red-purple, oedematous skin. (b) Large, deep ulcers with sharp edges on the medial surface of both labia minora covered by necrotic, greyish, fibrinous exudate. The arrows show the same ”kissing” ulcers that are labeled in Fig. 1a while the labia are in an opened position.
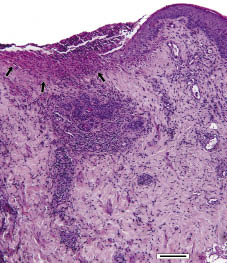
When considering the diagnosis, we discussed infections (syphilis, herpes simplex virus, HIV, chancroid), drug reactions, idiopathic or secondary aphthosis, Behçet’s syndrome, ulcus vulvae acutum, leukaemia, and pyoderma gangraenosum. Significant laboratory findings included: elevated levels of serum C-reactive protein (2.7 mg/dl (normal < 0.5 mg/dl)), atypical lymphocytosis (enlarged, activated lymphocytes, 13%), normal total white blood cell counts, and mild elevation of circulating liver enzyme levels (aspartate aminotransferase 79 U/l (< 33), alanine aminotransferase 132 U/l (< 35), γ-glutamyltransferase 70 U/l (< 38), lactate dehydrogenase 385 (< 250), and alkaline phosphatase 160 (< 150)). Hep-2 cells were positive for antinuclear antibodies at a titre of 1:2,560, showing chromosomal and centromeric distribution. However, no specific antigen was detected. Cultures for Haemophilus ducreyi, Neisseria gonorrhoeae and yeasts, analysis of a swab for herpes viruses (HHV-1, -2, and -3) by PCR, and serological tests for Treponema pallidum, human immunodeficiency viruses (HIV-1 and -2), herpes viruses (HHV-1, -2, -3, and -5) and hepatitis A and C viruses were negative. Varicella-zoster and hepatitis B serology confirmed prior vaccination. The EBV serology (performed on day 7) was positive for IgM against viral capsid antigen and IgG against early antigen. However, IgG antibodies against the viral capsid antigen and the nuclear antigen were absent. Low amounts of EBV were detected by PCR in a swab sample taken from the major ulcer on day 9. Direct immunofluorescent examination of a biopsy from the perilesional area of one of the ulcers showed fine, granular C3 complement deposition along the basement membranes and in the vessel walls, as well as vascular fibrinogen deposition. Immunoglobulin (IgG, IgM, IgA, IgE) and C4 complement deposition was not detected. Histological analysis of the lesional-perilesional skin on day 7 showed an ulcer covered by a fibrinoid crust with a neutrophil-dominated infiltrate. In the dermis, a dense perivascular lymphohistiocytic infiltrate was found, rich in neutrophils. The epidermis next to the ulcer showed acanthosis, mild spongiosis, a missing stratum granulosum, and a thin parakeratotic stratum corneum with some neutrophils and lymphocytes (Fig. 2). Yeasts were not detected by periodic acid-Schiff staining and no immunohistochemical evidence of HHV-1 and -2, EBV and T. pallidum infection was found. In situ hybridization using probes specific for EBV was negative. However, PCR amplification of DNA extracted from a formalin-fixed biopsy specimen using primers specific for EBV proved the presence of EBV DNA.
Fig. 2. Biopsy from the edge of one of the ulcers (H&E, 100× magnification). The ulcer was covered by a fibrinoid crust and infiltrated by neutrophils (arrows). The dermis displayed perivascular infiltration. The epidermis adjacent to the ulcer exhibited slight acanthosis, mild spongiosis, lack of stratum granulosum, and thin parakeratotic stratum corneum, as well as the presence of neutrophils and lymphocytes. Bar = 150 μm.
At the first visit, a short course of methylprednisolone was started (0.75 mg/kg body weight (40 mg) once a day for 4 days, and then tapered to zero within a week). Additionally, cefuroxime 500 mg twice daily and lidocaine cream (as necessary) were prescribed.
The ulcers healed within a month. No relapse occurred during the 2-year follow-up.
DISCUSSION
Ulcus vulvae acutum was first described by the German dermatologist Lipschütz in 1913 (4). No causative agent could be identified at that time. Lipschütz subdivided the condition into three major subtypes, based on clinical features (duration, systemic involvement, and relapse), corresponding in current terms most closely to genital herpes infection, Behçet’s syndrome, and the condition we now call ulcus vulvae acutum Lipschütz, which is probably a disease of polyaetiological origin. The association of some cases with EBV infection was suggested in 1977 by Brown & Stenchever (7) and in 1984 by Portnoy et al. (8). However, the exact aetiology and pathomechanism of Lipschütz’s ulcers are unknown. In addition to EBV, other infectious agents have been implicated, including cytomegalovirus, Mycoplasma pneumoniae and influenza A virus (6, 9–11). Idiopathic acute genital ulcers have also been described (12, 13). Since 1977, approximately 40 EBV-linked cases have been reported.
EBV-associated ulcus vulvae acutum (EBV-AUVA) presents typically as one or a few very painful, deep ulcers with vivid, purple-red edges in adolescent girls (5). At least one of the ulcers is usually larger than 1 cm. The age at which the incidence of EBV-AUVA peaks is identical to that of patients with infectious mononucleosis (5, 14). The genital disease is usually associated with systemic symptoms and lymphadenopathy distant from the inguinal region. Initially, most patients complain about one or two prodromal symptoms (e.g. fatigue, anorexia, headache, or low-grade fever), eventually developing characteristic symptoms of infectious mononucleosis, such as high fever, sore throat associated with pharyngotonsillitis, periorbital oedema, tender cervical or generalized lymphadenopathy, and/or hepato- and splenomegaly. No infectious agent other than EBV is usually detected. Histologically, EBV-AUVA is characterized by extensive, perivascular, mixed inflammatory cell infiltration resulting in severe, local, unspecific, necrotizing vasculitis (5, 15). EBV can be detected directly from lesions, in the blood, or in oropharyngeal lavage samples (5, 8). The results of serological and other laboratory tests performed when genital ulcers appear usually indicate acute infectious mononucleosis, but seroconversion may be delayed for several weeks.
In the present case, diagnosis was based on characteristic clinical symptoms, laboratory results, exclusion of infectious agents other than EBV, supporting histological results, serological evidence of acute infectious mononucleosis and, finally, direct detection of EBV in both a lesional swab sample and a biopsy specimen by PCR. Our case corresponds well with other clinical reports of EBV-AUVA. The healing time of 4 weeks was among the longest reported (mean 19 days, range 2–10 weeks) (13, 15).
It is now generally accepted that EBV-AUVA is not a sexually transmitted disease, although genito-genital transmission of EBV may be possible (16, 17). Most patients, like the young woman described here, report no sexual activity prior to the appearance of genital ulcer(s), whereas sexually active patients deny unsafe behaviour or apparent genital infection of their partner. In virgins, oral contact with saliva and resulting systemic disease is a much more likely cause than unreported sexual transmission. The genital area has been shown to be a reservoir for EBV in more than 10% of males and 20% of females (17). As a consequence, we speculate that the number of diagnosed EBV-AUVA cases would be several orders of magnitude higher if percutaneous sexual transmission were a prerequisite for EBV-AUVA. That the ulceration might be a complication of a systemic viraemia, even if the virus may have been initially transmitted via genital contact, is supported by the fact that EBV-AUVA has only been reported in association with viraemia, including acute, early infectious mononucleosis (5, 15). EBV might reach the site of ulceration via lymphocytic infiltration, haematolymphogenic circulation, or through autoinoculation with saliva or cervico-vaginal fluid.
To date, three hypotheses have been suggested for the aetiopathogenesis of EBV-AUVA (6, 8):
The first hypothesis is supported by direct evidence for immune complex formation and deposition in infectious mononucleosis (14, 18–20). The histological signs observed in EBV-AUVA are consistent with localized immune complex vasculitis. Free virus may be detected in swabs and biopsy specimens due to the general viraemia (during acute infection, all body fluids proably contain EBV DNA in quantities sufficient for detection by PCR) (21). However, viral DNA and proteins do not need to be present in keratinocytes or lymphocytes within the affected vulvar tissue, explaining the negative results of in situ hybridization and immunohistochemistry. Nevertheless, the failure to detect the virus within our patient’s tissue does not necessarily substantiate this hypothesis, as the negative result may purely reflect the limited sensitivity of the techniques used. This is also supported by in situ hybridization studies performed by Farhi et al. (13), in which three of four tissue samples were negative. In addition, signs of vasculitis in sites other than the vulva have not been identified in reported cases. In our patient, we were unable to detect immune complexes by direct immunofluorescence, whereas complement activation in the vessel walls and along the basement membranes may constitute unspecific signs of small vessel vasculitis.
The second hypothesis is based on: (i) clinical manifestation compatible with a severe human herpes virus infection (multiple painful necrotic ulcers and erosions, as typically seen in severe HHV-1 and -2 infections); (ii) evidence of the capability of EBV to replicate in both oropharyngeal and uterine cervical epithelial cells (both in vitro and in vivo during infectious mononucleosis) (22–24); and (iii) detection of EBV in genital skin (17). Infection of the cervical epithelium may occur directly through sexual transmission or indirectly through migration of B lymphocytes harbouring and shedding the virus (24). EBV replication in uterine cervical epithelial cells in vivo leads to the shedding of virus into the cervicovaginal fluid reaching levels sufficient for detection by lymphocyte transformation and cytohybridization assays (17, 24). Although some authors attributed the vulvar involvement to (auto-)inoculation with saliva (25) or the exclusive migration of lymphocytes into vulvar skin (15, 26), we assume that autoinoculation more likely occurs percutaneously due to the large numbers of free virus particles in the cervicovaginal fluid (i.e. without the need of genital contact with saliva). Our hypothesis is supported by the significant vaginal discharge seen in our patient, the observation of “kissing ulcers” in Lipschütz’s original description (4), in our patient (Fig. 1) and 12 other cases, and the distinct localization of the ulcers (Fig. 1 and 4–6, 8, 15, 25, 26). In all reported cases, the primary affected areas were the medial and outer surfaces of the labia minora around the vestibulum, which are extensively exposed to vaginal discharge. Involvement of the lateral labial surfaces, the deep folds between the outer and inner labia, the clitoris, and the perianal region has not been reported. Other authors have not mentioned either the absence or presence of vaginal discharge in their patients, but small levels (as frequently occur in primary herpes simplex infections) may be sufficient for epidermal infection. This hypothesis is challenged by the lack of data confirming the replication of EBV in vulvar keratinocytes and the clinical observation that, in contrast to herpes simplex or varicella-zoster infections, EBV-associated mucocutaneous erosions or ulcerations have not been described in immunocompetent patients other than those with EBV-AUVA, although EBV infection presents typically with palatal petechial enanthem (14). Finally, the in situ hybridization studies of Farhi et al. (13) showed that EBV may be present in mononuclear cells but not keratinocytes, suggesting that EBV-AUVA may be more likely to result from an indirect immune reaction than from a direct epithelial cytopathogenic effect of EBV.
According to the third hypothesis, EBV-AUVA may be the manifestation of an EBV-induced unipolar or bipolar major aphthosis (6, 27). Similar pathogenesis would explain ulcus vulvae acutum in association with other acute underlying diseases such as M. pneumoniae or influenza virus infections. However, there was no recurrent aphthosis in our patient’s history including the 2-year follow-up, and most other authors do not report recurrent aphthosis (13).
We assume that deep, necrotic ulcers are not an essential diagnostic criterion for EBV-AUVA. They might rather represent the more severe end of the spectrum of this disease, which may comprise a continuum with small abortive lesions (such as symptomless vulvar petechiae) or small superficial herpes simplex-like erosions being at the other end of the spectrum. Consequently, mild cases may be significantly underdiagnosed. We therefore propose diagnostic testing for EBV (together with cytomegalovirus and M. pneumoniae) in any vulvar disease resembling genital herpes simplex infection if the patient is a virgin adolescent, there is a concomitant upper respiratory disease, or specific HHV-1, -2, and -3 tests are negative.
Farhi et al. (13) and Huppert et al. (27), respectively reported the incidence of EBV-AUVA among acute genital ulcers in adolescent women to be approximately 30% and 10%. Careful diagnosis will help to unravel the true incidence of EBV-AUVA.
In our patient, anti-centromere and anti-chromosome antibodies were found in significant titres. Appearance of antinuclear antibodies and other autoantibodies (mainly cryoglobulins and anti-lymphocyte antibodies) is common in the early phase of infectious mononucleosis and may persist for years (14). Transition into manifest autoimmune disease is even possible (28). In most cases, these immunological phenomena are transient and disappear spontaneously.
Although EBV-AUVA is a self-limiting condition, supportive treatment (a short course of systemic glucocorticoids, management of severe pain, topical antiseptics and/or systemic antibiotic therapy) is essential. Antiviral drugs are ineffective (13, 29).
In conclusion, we describe herein an adolescent female virgin with multiple acute genital ulcers associated with infectious mononucleosis. EBV DNA was detected in the ulcers by PCR. However, the virus could not be visualized in the ulcer tissue. It is not known whether EBV-AUVA is caused by interplay between a direct cytolytic effect of EBV replication in the vulvar epithelium and the associated inflammatory reaction or whether it is due to a localized destructive hypersensitivity reaction to deposited immune complexes. Detectable virus particles in the ulcers may result from viraemia via infiltration of infected lymphocytes, haematolymphogenic circulation, or autoinoculation with saliva or cervicovaginal fluid. Further studies are needed to clarify the aetiology of what is a very rare manifestation of EBV infection and, perhaps, a special type of aphthosis.
REFERENCES
