Yasumasa Kanai, Takahiro Satoh, Ken Igawa and Hiroo Yokozeki
Department of Dermatology, Graduate School, Tokyo Medical and Dental University, Tokyo, Japan
Yasumasa Kanai, Takahiro Satoh, Ken Igawa and Hiroo Yokozeki
Department of Dermatology, Graduate School, Tokyo Medical and Dental University, Tokyo, Japan
Psoriasis is a chronic skin disease mediated by Th17 and/or Th1 cells. Tim-3 is a cell surface molecule preferentially expressed on Th17 and Th1 cells. The interaction of Tim-3 with Tim-3 ligand inhibits cytokine production. To assess whether T cells in psoriasis have functional abnormalities, expression of cell surface Tim-3 on blood T cells producing interleukin-17 (Th17/Tc17 cells) or interferon-γ (Th1/Tc1 cells) was examined by flow cytometry. Psoriasis patients had higher numbers of Th17 and Tc17 cells, as well as Th1 and Tc1 cells, than healthy donors. However, Th17, Th1 and Tc1 cells in psoriasis did not efficiently express Tim-3 upon activation, compared with those from atopic dermatitis and healthy donors. Tim-3– cells showed more potent cytokine production than Tim-3+ cells. Impaired Tim-3 expression allows Th17, Th1 and Tc1 cells to escape from Tim-3-mediated negative regulatory systems and may contribute to the pathogenesis of psoriasis. Key words: atopic dermatitis; psoriasis; Th1 cells; Th17 cells; Tim-3.
(Accepted September 22, 2011.)
Acta Derm Venereol 2011; 91: XX–XX.
Takahiro Satoh, Department of Dermatology, Graduate School, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, 113-8519, Japan. E-mail: tasa-1688.derm@tmd.ac.jp
Psoriasis vulgaris is a chronic, polygenic disease characterized by multiple erythematous plaques with scales. The complex etiopathogenesis of psoriasis is only partially understood. Interferon-gamma (IFN-γ)-producing Th1 cells have long been thought to be key players in the inflammation of psoriasis (1–3). However, levels of interleukin (IL)-23p19 are also elevated in the lesional skin of psoriasis (4), and local administration of IL-23 induces psoriatic skin inflammation in mice (4). IL-23 is a major cytokine that promotes expansion of Th17 cells (5); thus, it is now believed that Th17 cells, rather than Th1 cells, are a prerequisite for the development of psoriatic lesions. Nevertheless, Th1 cells and IFN-γ are elevated in psoriatic lesions (2, 6) and IFN-γ programmes antigen-presenting cells to induce IL-17+ T cells via IL-1 and IL-23 (7). IFN-γ also supports CCL20 and β-defensin 2 production by keratinocytes synergistically with IL-17 (7).
T-cell Ig- and mucin domain-containing molecule (Tim)-3 belongs to the TIM family, which has an Ig V-like domain and a mucin-like domain. Accumulating evidence in mice has revealed that Tim-3 is expressed in Th1 cells, but not in Th2 cells, and that the interaction between Tim-3 and Tim-3 ligand negatively regulates Th1 immune response (8–10). In addition, recent findings have demonstrated that murine Th17 cells express Tim-3 (11) and that Tim-3 provides negative regulatory signals in Th17 cells (12). Similarly, a study in human CD4+ T cells confirmed Tim-3 expression on activated Th1 cells and, to a lesser extent, on Th17 cells (13). Blocking of Tim-3 resulted in enhanced production of IFN-γ and IL-17 from CD4+ T cells. Human CD8+ T cells also express Tim-3, and patients infected with hepatitis C virus or human immunodeficiency virus-1 showed increased levels of Tim-3 on CD8+ T cells. Tim-3-expressing CD8+ T cells are regarded as dysfunctional T cells characterized by their low capacity for IFN-γ production compared with Tim-3 negative CD8+ T cells, and this could be a central event leading to an impaired immune defence against viral infection (14, 15).
A physiological ligand of Tim-3 is galectin-9, a tandem-repeat type of galectin that comprises two homologous carbohydrate recognition domains connected by a linker peptide (16, 17). Galectin-9 bound to Tim-3 on Th1 and Th17 cells induces cell apoptosis and/or suppresses cell differentiation (12). Galectin-9 expression is up-regulated by IFN-γ (18, 19) which may eliminate or down-regulate Tim-3-expressing Th1 and Th17 cells as part of a negative feedback mechanism; thus impairment of the galectin-9-Tim-3 network may disrupt immunological homeostasis, resulting in pathogenic states, such as autoimmune diseases (20).
We have demonstrated previously that galectin-9 expression is elevated in the lesional skin of psoriasis (21). Considering the predominance of Th17/Th1 inflammation, we hypothesized that the Tim-3-mediated braking systems in T cells are impaired in psoriasis. To this end, in the present study, Tim-3 expression on blood Th1 and Th17 cells was assessed by flow cytometry.
MATERIALS AND METHODS
Antibodies
Fluorescein isothiocyanate (FITC)-conjugated anti-human CD4 Ab (FITC-CD4, RPA-T4), FITC-CD8 (RPA-T8), PerCP-Cy5.5-IFN-γ (4S.B3) and PerCP-Cy5.5-IL-17 (eBio64DEC17) were purchased from eBioscience Inc. (San Diego, CA, USA). PE-Tim-3 (344823) was obtained from R&D Systems (Minneapolis, MN, USA).
Preparation of blood samples
Heparinized peripheral blood was collected from patients with psoriasis vulgaris (15 patients: 9 males, 6 females; mean age 56.1 years; age range 24–78 years) or atopic dermatitis (AD) (9 patients: 4 males, 5 females; mean age 35.8 years; age range 29–44 years) after obtaining informed consent. Psoriasis patients were controlled with topical corticosteroids and/or active vitamin D3 analogues. Five patients received narrow-band ultraviolet B (NB-UVB) phototherapy. Patients receiving systemic treatments, such as systemic corticosteroids, etretinate, cyclosporine and anti-tumour necrosis factor-alpha (anti-TNFα) therapy, were excluded. Patients with AD were treated with topical corticosteroids and/or systemic anti-histamines. Clinical severity scores for psoriasis (Psoriasis Area and Severity Index (PASI) score) and AD (Eczema Area and Severity Index (EASI) score) were 7.4 ± 3.74 and 17.1 ± 7.16 (mean ± standard deviation (SD)), respectively. All patients were confirmed as having extrinsic AD. This study was approved by the ethics committee of Tokyo Medical and Dental University. Control samples were obtained from 12 healthy volunteers (4 males, 8 females; mean age 28.7 years; age range 21–39 years). Peripheral blood mononuclear cells (PBMC) were isolated from the interface of density gradient centrifugation by Ficoll-PaqueTM PLUS (GE Healthcare, Uppsala, Sweden) at 1000 × g for 20 min at 25ºC.
Cell stimulation
PBMC were treated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St Louis, MO, USA), 500 ng/ml ionomycin (Calbiochem, San Diego, CA, USA) and 10 μg/ml Brefeldin A (Sigma-Aldrich) at 37ºC for 4 h.
Flow cytometry
PBMC suspended in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) (Sigma-Aldrich) (1×106 cells/100 μl/tube) were stained with antibodies against cell surface markers (CD4, CD8, and/or Tim-3) for 20 min on ice. For intra-cellular cytokine staining, cells were then treated with Cytofix/CytopermTM (BD Biosciences, San Jose, CA, USA), and were incubated with antibodies against cytokines (IL-17 or IFN-γ) for 20 min on ice. Lymphocytes were gated by forward and side scatter. Stained cells were analysed by FACSCaliburTM (BD Biosciences).
Statistical analysis
The Wilcoxon rank-sum test was used to assess the statistical significance of differences between mean values.
RESULTS
Increased frequency of T cells producing IL-17 and IFN-γ in psoriasis
PBMC were stimulated with PMA/ionomycin and the prevalence of cytokine-producing cells was assessed by flow cytometry. Psoriasis patients showed higher frequencies of IL-17+/CD4+ cells (Th17 cells), IFN-γ+/CD4+ cells (Th1 cells), and IFN-γ+/CD8+ cells (Tc1 cells) than healthy donors (Fig. 1). IL-17+/CD8+ cells (Tc17 cells) were also elevated in psoriasis patients, but the increase tended to be more marked in AD patients.
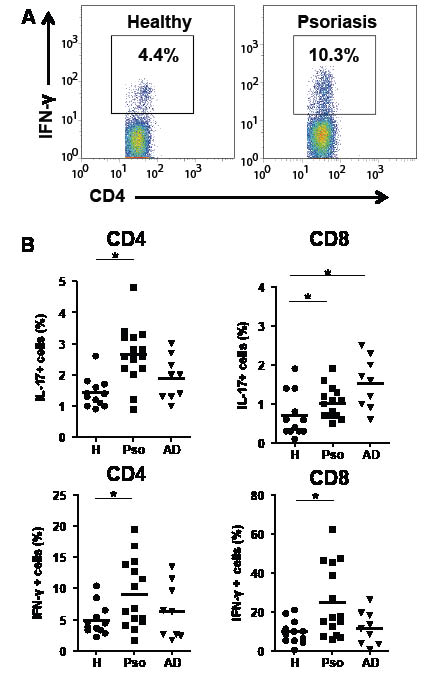
Fig. 1. Increase in cells producing interleukin (IL)-17 and interferon (IFN)-γ in psoriasis. Peripheral blood mononuclear cells (PBMC) were stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin for 4 h. Cells were stained with fluorescein isothiocyanate (FITC)-CD4 or -CD8 antibodies (Abs), followed by intracellular IL-17 and IFN-γ staining after permeabilization. (A) Representative features of flow cytometry. (B) Results of flow cytometric analysis. *p < 0.05 by Wilcoxon rank-sum test. H: healthy donors; Pso: psoriasis patients; AD: atopic dermatitis patients.
Tim-3 expression on CD4+ T cells and CD8+ T cells
In order to understand the regulatory systems of Tim-3 in psoriasis, we initially examined surface expression of Tim-3 on unstimulated T cells (Fig. 2). The prevalence of unstimulated CD4+ T cells expressing Tim-3 in psoriasis patients was slightly lower compared with healthy donors, and differences from AD patients were statistically significant. When the cells were stimulated in vitro with PMA/ionomycin, CD4+ T cells exhibited an increase in the expression of surface Tim-3. In this set of experiments, the prevalence of Tim-3+/CD4+ cells in psoriasis was significantly lower than in healthy donors and AD patients.
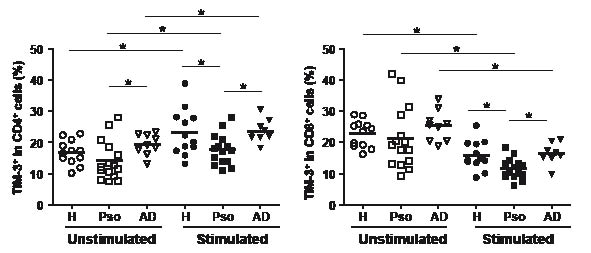
Fig. 2. Tim-3 expression on T cells from psoriasis. Tim-3 expression on CD4+ and CD8+ T cells were analysed by flow cytometry before (unstimulated) and after in vitro stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin (stimulated). *p < 0.05 by Wilcoxon rank-sum test. H: healthy donors; Pso: psoriasis patients; AD: atopic dermatitis patients.
In unstimulated CD8+ T cells, there were no appreciable differences in Tim-3 expression in the 3 groups. In contrast to CD4+ T cells, in vitro stimulation caused a reduction in Tim-3+ cells on CD8+ T cells. The prevalence of Tim-3+/CD8+ cells in psoriasis was again lower than in healthy donors and in AD patients.
Decreased Th17 cells and Th1/Tc1 cells expressing Tim-3 in psoriasis
We then aimed to verify Tim-3 expression on each T-cell subset. The prevalence of Tim-3+ cells in Th17, Th1 and Tc1 cells was significantly lower in psoriasis patients than in healthy donors (Fig. 3), despite the fact that psoriasis patients had higher numbers of these subsets of cells. We also observed a trend toward decreased frequency of Tim-3+ cells in Tc17 cells, although statistical significance was not observed.
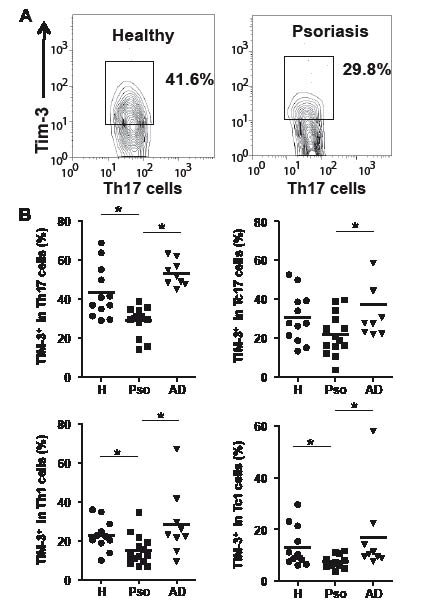
Fig. 3. Dysregulation of Tim-3 in Th17, Th1, and Tc1 cells from psoriasis. Tim-3 expression on CD4+ cells producing interleukin (IL)-17 (Th17) or interferon (IFN)-γ (Th1) and on CD8+ cells producing IL-17 (Tc17) or IFN-γ (Tc1) after stimulation was assessed. (A) Representative features of flow cytometry. PBMC were stimulated with PMA. CD4 (+)/IL-17 (+) cells were gated and Tim-3 levels on these cells were analyzed. (B) Results of flow cytometric analysis. *p < 0.05 by Wilcoxon rank-sum test. H: healthy donors; Pso: psoriasis patients; AD: atopic dermatitis patients.
No correlations were seen between Tim-3 expression in these Th/Tc cells and clinical PASI scores (Pearson's correlation coefficient test, data not shown).
Recent findings indicate that Tim-3– cells are more potent in producing cytokines than Tim-3+ cells (14, 15, 22). Consistent with these findings, in psoriasis patients, both CD4+ T cells and CD8+ T cells lacking Tim-3 had higher levels of cells producing the cytokines IL-17 and IFN-γ, than those expressing Tim-3 (Fig. 4).
DISCUSSION
Psoriasis is a chronic disease mediated by Th17 and/or Th1 immunity. Tim-3 provides negative regulatory signals in T cells. This study demonstrated dysregulated expression of Tim-3 on T cells producing IFN-γ and/or IL-17 from psoriasis patients.
Increased numbers of Th17 and Th1 cells were observed, as well as Tc17 and Tc1 cells, in peripheral blood from psoriasis patients, compared with healthy donors. These observations were consistent with a previous report that the prevalence of Th17, Th1 and Tc1 in lesional skin was higher in psoriasis than in AD, although their study did not detect statistical significance in peripheral blood T cells (6).
AD is considered to be a Th2- and/or Th1-mediated disease (23). However, a recent study reported that Th17 cells are elevated in the blood of AD patients, and that this elevation is correlated with disease severity (24). In the present study, we observed a trend toward increased frequency of Th17 cells in AD patients, but it was not statistically significant (Fig. 1). This was apparently caused by the variable distribution in the prevalence of Th17 cells among AD patients or by the small number of samples. In contrast, it was intriguing that AD showed a higher prevalence of Tc17 cells than healthy donors. Tc17 cells may thus contribute to the pathogenesis of AD rather than psoriasis. In mice, Tc17 cells play critical roles in the efferent phase of murine contact hypersensitivity, a model of eczematous reaction (25).
In vitro activation induced up-regulation of cell surface Tim-3 in CD4+ T cells (Fig. 2). This was in contrast to CD8+ T cells, where Tim-3 was down-regulated after stimulation. Although the actual regulatory mechanisms for the control of cell surface Tim-3 are unknown, these data indicate that CD4+ T cells and CD8+ T cells have different regulatory systems with regard to Tim-3.
The most notable finding in this study was that psoriasis patients had lower levels of Tim-3+ cells among Th17, Th1 and Tc1 cells (Fig. 3), although the actual contribution of Tc1 cells in psoriasis is uncertain. IL-17 and/or IFN-γ-producing T cells lacking Tim-3 could escape from negative regulatory systems mediated by Tim-3. Indeed, Tim-3– cells consisted of larger populations of IL-17 and IFN-γ-producing cells compared with Tim-3+ cells (Fig. 4).
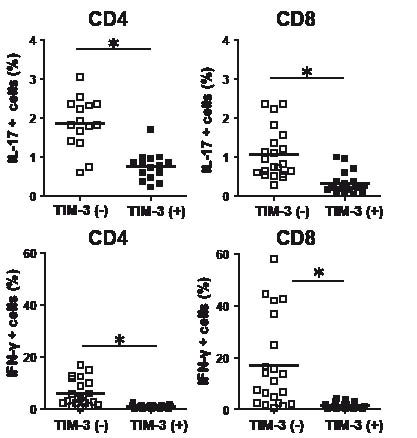
Fig. 4. Comparison of cytokine generation between Tim-3– and Tim-3+ cells from psoriasis patients. Intracellular interleukin (IL)-17 or interferon (IFN)-γ in Tim-3– and Tim-3+ cells were analysed after stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin. *p<0.05 by Wilcoxon rank-sum test.
Galectin-9 expression in lesional skin is elevated in psoriasis (21). The major source of galectin-9 in psoriatic skin appears to be fibroblasts (21), and this is consistent with the observations that in vitro stimulation of human dermal fibroblasts with IFN-γ induces galectin-9 expression (21) and Th1 immunity is predominant in psoriatic skin lesions. Nevertheless, in psoriasis, galectin-9 stimulated by Th1 cell-derived IFN-γ may not be able to eliminate Th1/Tc1 and Th17 cells because of their impaired expression of Tim-3; this could be one of central events leading to exacerbation of psoriatic inflammation. Dysregulated expression of Tim-3 on T cells has also been demonstrated in patients with multiple sclerosis (26).
The mechanisms underlying the dysregulation of Tim-3 in psoriasis are unknown, but may be caused by functional defects occurring during cell differentiation/expansion of T cells. Although the innate immune response mediated by plasmacytoid dendritic cells producing IFN-α may have a role in psoriasis (27, 28), we cannot provide a clear explanation for the link between plasmacytoid dendritic cells and Tim-3 dysregulation on T cells. Alternatively, there may be dysfunction in myeloid dendritic cells, such as TNF- and inducible nitric oxide synthase-producing dendritic cells, which promote Th1/Th17 cell development (29).
Collectively, T cells producing IL-17 and/or IFN-γ in psoriasis appear to be characterized by impaired expression of Tim-3, which mediates exacerbated activity of Th17/Th1 immunity because of the lack of braking signals through Tim-3. Further research is needed in order to clarify the molecular mechanisms causing disturbed Tim-3 expression of T cells in psoriasis.
The authors declare no conflicts of interest.
REFERENCES
