Satoshi Izaki1, Juniku Mitsuya1*, Tomoyoshi Okada1, Hiroshi Koga2, Takashi Hashimoto2 and Tadashi Terui1
1Division of Dermatological Science, Department of Dermatology, Nihon University School of Medicine, 30-1 Oyaguchi-Kamicho, Itabashi-ku, Tokyo 173-8610, and 2Department of Dermatology, Kurume University School of Medicine, and Kurume University Institute of Cutaneous Cell Biology, Kurume, Fukuoka, Japan. *E-mail: mitsuya.juniku@nihon-u.ac.jp
Accepted Jun 30, 2014; Epub ahead of print Jun 30, 2014
Linear IgA bullous dermatosis (LABD) is characterised by linear deposition of IgA along basement membrane zone (BMZ) in direct immunofluorescence (IF) (1). The term of linear IgA/IgG bullous dermatosis (LAGBD) was proposed for some cases, which showed IgA and IgG deposition to BMZ (2).
In LABD, indirect IF of 1 M NaCl-split normal human skin shows 3 distinct patterns (IgA reactivity on the epidermal side, on the dermal side and on both sides) called lamina lucida, sublamina densa and mirror image types, respectively (3).
In the lamina lucida type LABD, the most common antigen is LAD-1, the 120-kDa/97-kDa extracellular domain of BP180 (4). Autoantigens for sublamina densa type LABD have not been fully investigated. In the previously reported cases, 2 cases reacted with type VII collagen (5, 6), and 1 case reacted with laminin γ1 (7). However, no IgA autoantibodies to laminin-332 have been reported.
Here we present a rare case of LAGBD, in which IgA and IgG autoantibodies reacted with the dermal side in indirect IF and with laminin-332 in immunoblotting. To the best of our knowledge, this is the first report of LAGBD with anti-laminin-332 autoantibodies.
CASE REPORT
A 53-year-old Japanese man visited us in September 2011, complaining of a 2-month history of skin lesions. Physical examination revealed generalised erythematous skin lesions with tense bullae and erosions, as well as pustules, crusts, and scales (Fig. 1a, b). Some of these skin lesions formed an annular pattern 2–6 cm in diameter (Fig. 1c). Mucous membranes were not involved. Histopathology of the skin biopsy specimen taken from the bullae on the left upper arm showed a subepidermal blister with numerous neutrophils and a few eosinophils.
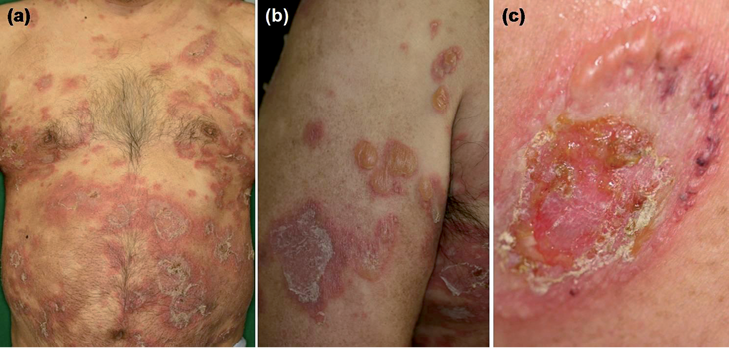
Fig. 1. Multiple annular erythemas with vesicles, bullae and scales were seen on the trunk (a) and extremities (b). Vesicles and bullae appeared in an annular arrangement (c).
Direct IF for IgG, IgA, IgM and C3 was performed on perilesional skin biopsy specimens. Indirect IF was performed on normal human skin and 1 M NaCl-split normal skin as described previously (8). Direct IF for the skin biopsy revealed a linear BMZ deposition of IgG and IgA, and C3. Indirect IF demonstrated anti-BMZ autoantibodies of IgG class (titre 1:40), but not IgA class (data not shown). Indirect IF of 1 M NaCl-split skin detected IgG reactivity with dermal side (titre 1:10), while no IgA reactivity was found.
Immunoblot analyses were performed using epidermal and dermal extracts from normal human skin, recombinant proteins of NC16A- and C-terminal domains of BP180, and purified human laminin-332, as well as concentrated HaCaT cell culture supernatant, which contains the 120-kDa LAD-1, soluble ectodomain of BP180. Immunoblot analysis showed exclusive IgA and IgG reactivity with the 165-kDa and 145-kDa forms of α3 subunit and the 105-kDa γ2 subunit of laminin-332 (Fig. S11). The titre of our patient was much lower than that of control serum from anti-laminin 332 mucous membrane pemphigoid (MMP) patient. There was no reactivity in other immunoblot analyses.
Based on these observations, we diagnosed the patient as LAGBD with anti-laminin-332 autoantibodies. The patient was initially treated with combination of doxycycline hydrochloride (200 mg/day) and nicotinamide (900 mg/day) without sufficient improvement. When dapsone (75 mg/day) was added on day 7, the skin lesions improved quickly within a few days leaving milia formation. This treatment was continued without recurrence.
About one and half years later, no circulating IgA and IgG autoantibodies were detected by indirect IF of 1 M NaCl-split skin, and IgA and IgG reactivity with laminin-332 in immunoblotting disappeared.
DISCUSSION
Our patient without mucosal involvement should be differentiated from IgA type anti-laminin-332 MMP, only 3 cases of which have been reported thus far (9–11). Two cases had severe mucous membrane involvement and were refractory to treatments (9, 10), while another case had only ocular lesion (11).
Our patient showed clinically papules, scales, and bullae on annular erythemas, and histopathologically subepidermal bulla with numerous neutrophils. In addition, he improved quickly after treatment with dapsone. Therefore, we diagnosed this case as LAGBD with anti-laminin-332 autoantibodies.
Two cases of sublamina densa-type LABD were reported to show IgA autoantibodies to the 290-kDa type VII collagen, which may be diagnosed as IgA-type epidermolysis bullosa acquisita (EBA) (5, 6). However, the clinical and histopathological features in these 2 cases were considerably different from those of EBA. Therefore, although IgG autoantibodies in EBA patients react mainly with NC1 domain of type VII collagen, IgA autoantibodies in the 2 LABD cases might react with different epitope on type VII collagen (5, 6). Likewise, epitope on laminin-332 for IgA autoantibodies in our patient may differ from that of anti-lamininn-332 MMP.
Laminin-332 is located to the dermal side by indirect IF of 1 M NaCl-split skin (12). Based on the traditional classification, our case is regarded as a ‘sublamina densa type’ of LAGBD. However, this denomination is not appropriate because laminin-332 is found at the lamina lucida-lamina densa interface (12). Thus, we would like to propose an alternative denomination, ‘sublamina lucida type’.
Abnormal IgA-antigen complexes in tissues induce the release of leukotriene B4 through cross-linking of FcαRI. This induces sustained recruitment, constant activation and infiltration of granulocytes, which cause severe tissue damage and aggravation of the symptoms of IgA autoantibody-mediated autoimmune diseases (13).
The pathogenesis of IgG autoantibodies has not been fully discussed in LAGBD reports thus far. In one report, passive transfer of IgG anti-laminin 332 autoantibodies to neonatal mice induced blisters (14). In our case, neither circulating IgA nor IgG autoantibodies were detected after clinical symptoms disappeared following treatments with dapsone. These observations suggest that not only IgA autoantibodies but also IgG autoantibodies are pathogenic.
Dapsone may bind to IgA directly and inhibits the adherence of neutrophils to anti-BMZ antibodies (15). However, it is unclear whether dapsone leads to disappearance of circulating IgG and IgA autoantibodies.

1http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1923
REFERENCES
