Chunxiao Wan, MD, PhD1,2, Jianan Li, MD3, Sheng Bi, MD, PhD2, Yan Zhao, PhD3 and Aicui Lin, PhD3
From the 1The Department of Rehabilitation Medicine,Tianjin Medical University General Hospital, Tianjin, 2Rehabilitation Medicine Centre, Chinese PLA General Hospital, Beijing and 3Rehabilitation Medicine Centre, The First Affiliated Hospital with Nanjing Medical University, Nanjing, China
OBJECTIVE: To locate and trace endogenous endothelial progenitor cells (EPCs) in rabbits subjected to myocardial ischaemia and/or physiological ischaemia training.
METHODS: Rabbits were randomly divided into 4 groups: a myocardial ischaemia group (subjected to myocardial ischaemia only); a physiological ischaemia training group (subjected to physiological ischaemia training only); a physiological ischaemia training-myocardial ischaemia group (subjected to both myocardial ischaemia and physiological ischaemia training); and a sham-operated group. Myocardial ischaemia was induced experimentally by a 2-min ischaemia, followed by a 1-h reperfusion. Physiological ischaemia training involved a 4-min isometric contraction elicited by electrical stimulation (biphase square wave, 40 Hz, 1 ms), which generated a contraction force at 40% of the maximal isometric contraction force. Myocardial ischaemia and/or physiological ischaemia training were performed twice a day, 5 days a week for 4 weeks. Capillary densities and EPC levels in both blood and the ischaemic heart region were then measured. EPCs were traced by double-labelling with super paramagnetic iron oxide and chloromethyl-benzamidodialkylcarbocyanine.
RESULTS: EPC levels in the blood and the ischaemic heart region both improved significantly in the physiological ischaemia training-myocardial ischaemia group (mean 0.046% (standard deviation (SD) 0.007), 0.013% (SD 0.005)) and group myocardial ischaemia (mean 0.038% (SD 0.016), 0.008% (SD 0.004)). For the physiological ischaemia training group, moderately raised EPCs were found in the blood (0.026 ± 0.010%), but not in the heart. Capillary density increased in the physiological ischaemia training-myocardial ischaemia and myocardial ischaemia groups. The dual-labelled EPCs were confirmed in the ischaemic heart region. Pearson’s analysis demonstrated that there is a positive correlation between EPC levels in the blood and the heart region (p < 0.05), and between circulating EPCs and the capillary (p < 0.05) for the physiological ischaemia training-myocardial ischaemia group.
CONCLUSION: Physiological ischaemia training can effectively improve endogenous EPCs. Their homing process from the circulating blood to the ischaemic myocardium was clearly traced in this study on rabbits. This homing process is of great importance for remote neovascularization.
Key words: physiological ischaemia training; angiogenesis; myocardial ischaemia; endothelial progenitor cell; homing.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Jianan Li, Rehabilitation Medicine Centre, First Affiliated Hospital of Nanjing Medical University. Nanjing 210029, Jiangsu Province, China. E-mail: lijianan@carm.org.cn
Accepted Jun 23, 2014; Epub ahead of print Sep 15, 2014
INTRODUCTION
Cardiovascular disease is the leading cause of death worldwide. By 2030, it is estimated that 40.5% of the US population will develop some form of cardiovascular disease (CVD). Between 2010 and 2030, the total direct medical costs of CVD in American are projected to triple, from $273 billion to $818 billion (1). The prevalence of CVD in China has increased a great deal during recent years, with 3 million deaths annually. Thus, effective prevention strategies are essential. Cardiac rehabilitation (CR) is a medically supervised programme that helps improve the health and well-being of people with heart problems. It could prevent heart disease, reduce cardiac deaths, and shorten hospital stay by improving recovery after myocardial ischaemia (MI). The current international practice guidelines strongly recommend referrals for CR after acute MI (I, A), coronary artery bypass surgery or percutaneous coronary intervention (I, A), chronic angina (I, B) and/or peripheral artery disease (I, A) (2).
CR was defined in the 1960s as an activity to help patients regain their normal health status following a cardiac event (3). Traditionally, CR can improve exercise tolerance and quality of life via aerobic exercise (4). Recently, it was reported that there was a “dose–response” relationship between the duration of CR sessions and long-term mortality (5). Several studies revealed that high-intensity aerobic exercises augmented vascular collateralizations in patients with CVD via an increase in endothelial progenitor cell (EPC) numbers (6–8). However, its clinical application is limited because of potential cardiac events, such as angina, arrhythmia, myocardial infarction and sudden cardiac death. Therefore, in many cases, a safe approach needs to be developed.
In our laboratory, physiological ischaemia training (PIT) was proposed as a safe approach to increase EPCs. PIT involves the isometric contraction of normal skeletal muscles, induced by electrical stimulation. Ischaemia is induced when the intramuscular pressure is equal to or larger than the arterial pressure. Related studies have shown that short maximum voluntary isometric contractions are safe for patients with severe heart failure (ejection fraction of approximately 20%) (9, 10), with no significant influence on blood pressure (11). Our previous study showed that PIT had little influence on blood pressure or heart rate (12).
In our previous studies, we reported that PIT on the remote normal limb could increase capillary density in the other limb (13) or the ischaemic heart region (12) and improve left ventricle ejection fraction and decrease infarct size (14). We also revealed that PIT could improve EPC number and migration (12). In addition, we demonstrated that isometric exercise increases collateral flow (15). However, direct evidence of endogenous EPC migration into local ischaemic tissues has not been observed.
It is postulated that EPCs may contribute to angiogenesis in response to ischaemic insults. They may target ischaemic areas, differentiate into endothelial cells, form new vessels, improve local reperfusion (16) and decrease infarct size (17). Therefore, EPCs may play an important role in our study. We assume that PIT could stimulate endogenous EPCs and contribute to angiogenesis in the remote ischaemic heart region. In this paper, the mobilization and neovascularization of endogenous EPC due to PIT is investigated in detail.
MATERIAL AND METHODS
This study focuses on 2 areas: (i) EPC homing and (ii) EPC tracing. A total of 36 adult male New Zealand white rabbits (2.5–3.0 kg, 3 months old) were recruited and randomly divided into 6 groups. Four groups were randomly selected for the EPC homing study and the remaining 2 groups were used for the EPC tracing study.
The study procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes for Health (NIH Publication No. 85-23, revised 1996) and approved by the ethics committees of Nanjing Medical University and Jiangsu Province Hospital.
Model of reversible intermittent myocardial ischaemia
A model of reversible intermittent MI was applied, as described by Gu et al. (18). Rabbits received 3% (v/v) sodium pentobarbital (1 ml/kg body weight) via a marginal ear vein. Supplemental anaesthesia was provided during the course of the experimental procedure as necessary. After the heart was exposed via a left anterior thoracotomy, a balloon occluder was implanted into the left ventricular branch. The success of the model was confirmed by ST-segment fluctuations over 0.1 mV, induced by the inflation and deflation of the inserted balloon. After the induction of experimental ischaemia, animals were placed in a cage for recovery.
Sham surgery involved the same cardiac exposure without placing the coronary occluder. Penicillin (4 × 105 U/day) was administered intramuscularly for 5 days post-surgery.
Model of physiological ischaemia training
The PIT model, based on isometric contractions induced by electrical stimulations, has been described previously (12–14, 19–20). The sciatic nerve of the right hind limb was exposed under anaesthesia. A platinum wire electrode was inserted longitudinally into the epineurium of the sciatic nerve and sutured in. The reference electrode was implanted into the ipsilateral gluteus maximus. Wires from these electrodes were taken under the skin to the nuchal region and connected to an external stimulator (SY-708A; Suyun, China). After surgery, rabbits were in recovery for 7 days before training.
The maximal isometric contraction force for each rabbit was assessed before training, as described in references (14, 19, 20). A strain gauge was fixed on the distal hind limb to measure the isometric contraction force. Isometric contractions were elicited by stimulation of the sciatic nerve with biphase square-wave impulses (1 ms, 40 Hz). The current intensity was gradually increased to reach the maximum isometric contraction force. Meanwhile, the current intensity corresponding to the contraction force at 40% maximal isometric contraction force was recorded, since such contraction force level had been proved to be an optimal parameter to increase local collateral circulation (19) and local venous lactate levels (13). During the PIT, each rabbit was performed isometric contraction at 40% maximal force induced by electrical stimulation (biphase square wave, 40 Hz, 1 ms) with the recorded corresponding current intensity for 4 min, twice a day, 5 days/week for 4 weeks.
Study design
Part 1 – Endothelial progenitor cell homing. Twenty-four rabbits were randomized into 4 groups: PIT-MI (with both PIT and MI), MI (with MI only), PIT (with PIT only), and SHAM (sham-operated). There were 6 rabbits per group. MI was induced with a 2-min ischaemia followed by a 1-h reperfusion, while PIT was performed through a 4-min isometric contraction at 40% maximal isometric contraction force. MI and/or PIT were performed twice a day, 5 days a week for 4 weeks. During the entire procedure, rabbits were observed to be fairly quiet without exhibiting signs of pain. Chest compressions, epinephrine, atropine and lidocaine were prepared for the treatment of malignant arrhythmia and cardiac arrest.
Peripheral blood-derived EPC (PB-EPC) levels were measured at baseline and the 1st, 2nd, 3rd and 4th weeks. At the end of the experiment, animals were sacrificed, and the EPC level in the ischaemic heart region (IH-EPCs) and the capillary density were measured.
Quantification of endothelial progenitor cells. EPCs were counted using a fluorescence-activated cell sorter (FACS, FACSCalibur, Becton Dickinson, USA). Mononuclear cells were isolated from 5 ml of peripheral blood by density gradient centrifugation using Histopaque-1077 (Sigma Diagnostics, St Louis, MO, USA). The ischaemic tissues were mechanically minced. Mononuclear cells from either the peripheral blood or myocardium homogenates were incubated for 30 min on ice with phycoerythrin (PE)-conjugated anti-mouse CD34 (BD Biosciences, San Jose, CA, USA) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse Flk-1 (R&D Systems, Minneapolis, MN, USA). Data were analysed using CellQuest software (Becton Dickinson, San Jose, CA, USA). All stains were isotype-matched to control antibodies from BD Pharmingen (San Diego, CA, USA). At least 1 × 106 cells were used for each experiment. CD34+/Flk-1+ cells were evaluated in the lymphocyte/monocyte population and expressed as a percentage of gated cells.
Determination of capillary density supply. Hearts were harvested and frozen in an optimal cutting temperature compound (Miles, Elkhart, IN, USA). They were cut into transverse sections, approximately 5 µm thick from apex to base. For capillary detection, sections were fixed overnight in 10% formalin, embedded in paraffin, and stained with mouse anti-CD31 antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Part 2 – Imaging of endothelial progenitor cell homing. For the tracking of EPC mobilization, autologous EPCs were cultured and expanded. After double-labelling with super paramagnetic iron oxide (SPIO) and a red fluorescent dye, chloromethyl-benzamidodialkylcarbocyanine (CM-Dil), EPCs were intravenously transplanted. A 7.0-T magnetic resonance imaging (MRI) scanner (PharmaScan, Bruker MA, Germany) was used to detect the images of the hearts ex vivo at 3 days after transplantation. Perls’ Prussian blue staining and immunofluorescence analysis were used for further confirmation.
Cell preparation. A total of 20 ml blood was drawn and mononuclear cells were isolated by density gradient centrifugation. Two million mononuclear cells were cultured in endothelial basal medium (EBM-2) supplemented with EGM-2MV SingleQuots (Lonza, Cologne, Germany). Four days later, non-adherent cells were removed and adherent cells were cultured continuously with fresh media replaced every 3 days.
Seven days later, adherent cells were confirmed as EPCs by double staining with Dil-labelled acetylated low-density lipoprotein (Dil-Ac-LDL, Molecular Probes, Carlsbad, CA, USA) and FITC-labeled Ulex europaeus agglutinin-1 (FITC-UEA-1) (Sigma, USA), as previously described (10). Double-stained cells were identified as EPCs.
After identification, third-passage EPCs were incubated with an SPIO solution (25 µg/ml) for 24 h at 37°C in 5% CO2. The cells were washed and labelled using CM-Dil (2 µg/ml, Molecular Probes, Eugene, Oregon, USA) for 5 min at 37°C, and then for 15 min at 4°C. Labelled cells were washed and resuspended for subsequent administration. SPIO was kindly donated by the Bioengineering Department of Southeast University in China.
Cell transplantation. Seven days after the balloon constrictor implantation, rabbits were injected with 2 ml of phosphate-buffered saline (PBS) containing 2 × 107 labelled EPCs into a marginal ear vein, while the other rabbits received 2 ml PBS intravenous injection. After transplantation, all rabbits began training. On day 3, rabbits were sacrificed and hearts harvested for 7.0T MRI scanning.
7.0 T magnetic resonance imaging. The hearts were harvested and scanned using a 7.0T horizontal-bore MR scanner with a 38-mm birdcage RF coil (PharmaScan, Bruker MA, Germany). Short-axis images of the heart were acquired over the entire left ventricle (FLASH-2d sequence; parameters: TR = 471 ms, TE = 5.0 ms; matrix = 256 × 256; maximal in-plane resolution = 156 × 156 µm2; NEX = 10, FOV = 4.0 × 4.0 cm2; slice thickness = 1 mm, 20 frames per cardiac cycle).
Statistical analysis
Data are expressed as mean and standard deviation (SD). Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test and were compared between groups using a 2-way analysis of variance (ANOVA), followed by a least significant difference post hoc test. The Pearson’s product moment correlation analysis was used to determine the relationship between PB-EPCs and IH-EPCs and between PB-EPCs and the capillary density. An α of < 0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.1.3 for Windows.
RESULTS
Thirty-six rabbits were used in this study: 24 were included in Part I for the EPC homing portion and 12 were included in Part 2 for the EPC tracing study. All animals survived and completed the experiment successfully.
Effects endothelial progenitor cell-endothelial progenitor cell levels
The numbers of CD34+/Flk-1+ PB-EPCs in PIT-MI, PIT, MI and SHAM groups are shown in Fig. 1A. At baseline, there are negligible differences in PB-EPC counts among all groups (p > 0.05). EPC counts increased in groups PIT-MI (0.046%, SD 0.007%, p < 0.01), MI (0.038%, SD 0.016%, p < 0.05) and PIT (0.026%, SD 0.010%, p < 0.05). They maintained high levels to the end-points. Group PIT-MI showed the highest level of EPCs compared with that of the other groups (p < 0.001, Fig. 1A). The peak value occurred at the 1st week in groups PIT-MI and MI. The peak value was delayed to the 2nd week in group PIT. Group SHAM showed no significant change throughout all periods (p > 0.05).
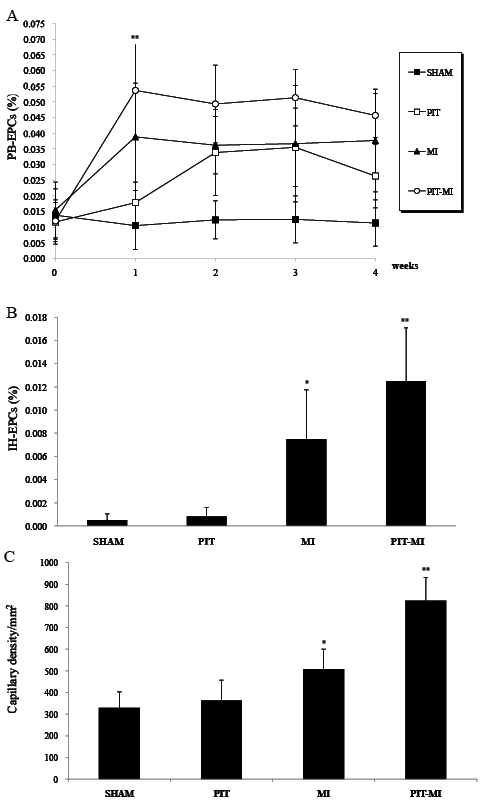
Fig. 1. (A) Peripheral blood-derived (PB)-endothelial progenitor cells (EPCs) for all groups. EPCs in group physiological ischaemia training (PIT)-myocardial ischaemia (MI) peaked in the first week and were maintained. EPCs in group MI increased similarly as in group PIT-MI, but at a lower level. EPCs in group PIT peaked in the second week. Little change was shown in the SHAM group (*p < 0.05, **p < 0.01). (B) At the end of the fourth week, ischaemic heart (IH)-EPCs increased in the MI group and more in the PIT-MI group. There was negligible difference between IH-EPCs in the SHAM and PIT groups (*p < 0.01, **p < 0.001). (C) Capillary densities were measured in the 4 groups; results are means ± standard deviations (SD). *p < 0.05 vs the SHAM group; **p < 0.01 vs the SHAM group.
Effects on ischaemic heart-endothelial progenitor cell levels
EPCs, defined as double-positive cells from homogenates in the ischaemic fraction of the heart, showed the phenotypes of CD34/Flk-1 and were quantified by FACS. At end-points, compared with SHAM (0.001%, SD 0.001%), the numbers of EPCs were significantly greater in groups PIT-MI (0.013%, SD 0.005%, p< 0.001) and MI (0.008%, SD 0.004%, p < 0.05, Fig. 1B). There was a negligible difference between group PIT and group SHAM (p > 0.05).
Capillary density assessment
At the end-points, compared with group SHAM, the capillary density at the ischaemic heart region had a 150% increase in group PIT-MI and a 54% increase in group MI (shown in Fig. 1C). The capillary density in group MI was lower than that in group PIT-MI and higher than that in the other 2 groups.
Endothelial progenitor cells double-labelling
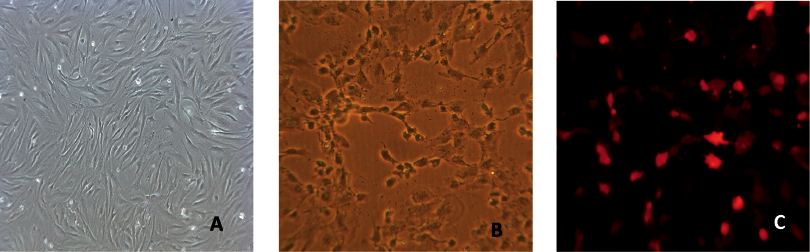
EPCs were first confirmed by the uptake of Dil-Ac-LDL and the binding of FITC-UEA-1, which were demonstrated by fluorescent microscopy by the emissions of red and green light (12).
For tracing, EPCs were dual-labelled with SPIO and CM-Dil, as shown in Fig. 2. EPCs took up SPIO by endocytosis during a 24-h in vitro culture (Fig. 2B) and bound with CM-Dil (Fig. 2C).
Magnetic resonance imaging of the transplanted endothelial progenitor cells
The transplantation model was applied to obtain direct evidence of enhanced EPC incorporation in the foci of neovascularization at the ischaemic site. Serial MRI (7.0 T) examinations were used to track the cells on the third day post-injection. The implanted SPIO-EPCs could be clearly visualized by hypointensities (black spots) in the 7.0T MRI images (Fig. 3A); however, these “black spots” were not detected in the corresponding sites of the control groups (Fig. 3B).
Histology of transplanted endothelial progenitor cells
After scanning with the 7.0T MRI, 5-µm-thick cryosections of the heart were stained with Prussian blue for iron detection. Perls’ Prussian blue stain-positive cells were found in the area where the signal intensity decreased (Fig. 3C). In region C of Fig. 3A, both SPIO-labelled and CM-Dil-labelled EPCs were detected (Fig. 3C, Fig. 3E). However, Dil- and SPIO-labelled EPCs were not found in region D of Fig. 3A (Fig. 3D). EPCs were co-cultured with SPIO; iron particles in cells were confirmed by Prussian blue staining (Fig. 3F). These findings indicated the phenomenon of EPCs targeting ischaemic myocardial tissues.

Relationship between peripheral blood-derived-endothelial progenitor cells and ischaemic heart-endothelial progenitor cells with capillary density
Potential relationships of PB-EPCs and IH-EPCs and the capillary density were examined. It was found that an increase in PB-EPCs was positively correlated with an increase in IH-EPCs in groups PIT-MI and MI (Fig. 4). Positive correlations of PB-EPCs with the capillary density in the ischaemic heart region in groups PIT-MI and MI were found (r = 0.81, p < 0.05 in group MI; and r = 0.84, p < 0.05 in group PIT-MI, Fig. 5). The data suggests that an increase in PB-EPC numbers could lead to an increase in IH-EPC population and a subsequent increase in capillary density.
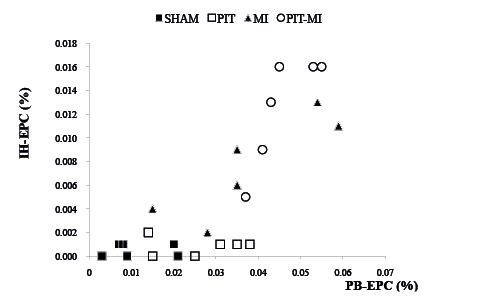
Fig. 4. Positive correlations between peripheral blood-derived (PB)-endothelial progenitor cells (EPCs) and ischaemic heart (IH)-EPCs in the experiment (group physiological ischaemia training (PIT)-myocardial ischaemia (MI): r = 0.845, p < 0.05; group MI: r = 0.857, p < 0.05). (B) Positive correlation of PB-EPC counts with capillary densities in groups MI and PIT-MI. MI: r = 0.812, p < 0.05; PIT-MI: r = 0.844, p < 0.05.
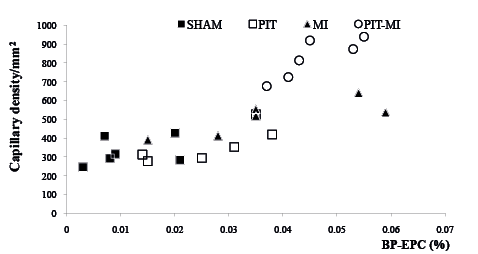
Fig. 5. Positive correlation between peripheral blood-derived- endothelial progenitor cell counts and capillary densities in groups myocardial ischaemia (MI) and physiological ischaemia training (PIT)-MI. MI: r = 0.812, p < 0.05; PIT-MI: r = 0.844, p < 0.05.
DISCUSSION
This study investigated and traced endogenous EPCs triggered by PIT. In Part 1, the data suggested that PIT could increase PB-EPC and IH-EPC numbers and capillary density. Increased numbers of IH-EPCs were detected in group PIT-MI, while fewer IH-EPCs were detected in groups PIT and SHAM. Statistical analysis showed positive correlations between PB-EPCs, IH-EPCs and the capillary density. In part 2, EPC mobilization was traced by an MRI scan and histology; results indicated that peripheral EPCs could migrate into the ischaemic heart region, which contributed to the local angiogenesis.
It has been demonstrated that there is an increase in the circulating EPC level during physical training (21). It was reported that a 3-week exercise training after acute myocardium infarction leads to a significant mobilization and increase in the functional activation of circulating progenitor cells (22). Laufs et al. (23) recorded that EPC levels elevated with 30-min intensive or moderate exercise. Previously, we had found that PIT could enhance PB-EPC numbers and migratory capacity (12). In this study, we disclosed that PB-EPC numbers increased the response to both myocardial ischaemia (MI) and PIT. The data illustrated that PB-EPCs in group PIT-MI peaked during the first week of the experiment, significantly more than in the other groups, which indicates positive interaction effects on PB-EPC numbers.
EPCs in ischaemic heart tissues were also determined in this paper. A significant increase in IH-EPCs was found in groups MI and PIT-MI, especially in the latter. In groups SHAM and PIT, there were negligible detectable EPCs in the same region. Pearson’s correlation analysis showed that there was a positive relationship between PB-EPCs and IH-EPCs, which means that increasing PB-EPCs may lead to the increase in IH-EPCs in the local myocardium.
This study used MRI scans and a fluorescent tracer technique to trace EPC mobilization. EPCs were labelled with SPIO and CM-Dil and were intravenously transplanted. The labelled EPCs in the ischaemic heart region were examined by 7.0T MRI and morphology. SPIO and MRI scans were explored as a novel safe and sensitive technique for cell tracing. The technique was applied in stem cell experiments as a molecular image approach (24–26). MRI, with its high spatial resolution, is an ideal method for in vivo cell tracking. Tagging cells with SPIO could induce sufficient MR cell contrast for in vivo imaging. Moreover, SPIO is biologically compatible, has low toxicity and does not affect cell viabilities. The SPIO used in our study was donated by the Bioengineering Department of Southeast University in China, and had been demonstrated to be effective for EPC labelling with no effect on cell proliferation and activity (27). The appropriate SPIO concentration was determined to be 25 µg/ml for EPC labelling (27). The intracytoplasmic iron was observed to leak from dead cells, resulting in hypointensity regions in MRI scans. To overcome this issue, we used CM-Dil to identify the presence of cells in the black-spots of the images. EPCs were confirmed if these regions were positive for both SPIO and CM-Dil. In the experiments, we observed SPIO- and CM-Dil-positive EPCs in the ischaemic heart region.
Our laboratory first proposed the concept of PIT as a potential approach for heart disease treatment. In this experiment, PIT entailed the electrical stimulation-induced isometric contraction of the normal limb. Isometric contractions were induced with electrical stimulation because animals could not voluntarily perform isometric contractions on demand. Ischaemia, which was induced during isometric contractions, could yield endogenous stem cells and contribute to neovascularization by the homing properties. In our experiments, ischaemia was termed physiological hypoperfusion because of its transient characteristics without tissue impairment (14). This study shows that PIT could generate a “release-for-remote demand” platform and reduce pathological changes. It also provides an alternative approach to promoting angiogenesis by mobilizing endogenous EPCs via limb isometric contraction, which would be valuable for improving cardiovascular disease treatment, even in the acute stage. This procedure could be useful for both primary and secondary preventions in cardiac intervention.
PIT exhibits the remote effects of limb ischaemia, but it is distinguishable from remote ischaemic preconditioning (RIPC). It was reported that non-invasive limb ischaemic stimulus, such as RIPC, could provide cardiac protection. Studies have been conducted wherein the non-invasive limb ischaemic stimulus was performed with 4 cycles of 5 min of limb ischaemia followed by a 5-min reperfusion. Ischaemia was achieved by the inflation of a blood pressure cuff to 25 mmHg above systolic blood pressure (28, 29). RIPC is a phenomenon whereby short periods of ischaemia and reperfusion of a tissue or organ (e.g. mesentery tissue, kidneys) can protect a distant tissue or organ (e.g. the heart) against subsequent potentially lethal ischaemia. It has a biphasic pattern of myocardial protection. An early classic phase is believed to act within a few min to 2 h after the preconditioning stimulus (30, 31). A delayed second window of protection occurs at 24–72 h (32, 33).
There are 3 main differences between PIT and RIPC: the objective, stimulus time, and duration of effects. As we know PIT aims to improve neovascularization, while RIPC protects the ischaemia-reperfusion injury. PIT requires a long period (4 weeks in our experiment), while RIPC has a relatively shorter duration. The effect of PIT is maintained at high levels for 4 weeks, while those of RIPCs only last for 72 h (32, 33).
The positive relationship between PB-EPCs and the capillary density was revealed in this study. However, the effect of EPC concentration on angiogenesis remains unclear. Many studies have demonstrated that an EPC increase could lead to angiogenesis (34). However, very few studies have examined the relationship between EPC concentrations and angiogenesis. This is probably because angiogenesis is influenced by several factors, such as delivery approach, local microenvironment, cytokines, the host age and sex and the history of acute or chronic diseases (35–40), and is not easy to analyse. This summarizes the limitations of the study; the relationship of EPCs and angiogenesis should be investigated in future studies.
In conclusion, our findings suggest that PIT could induce endogenous EPCs and augment the levels of PB-EPCs and IH-EPCs, which eventually contributed to remote angiogenesis. The study provides a meaningful process for both primary and secondary preventions in cardiac intervention. In addition, this study may offer a novel and safe approach for treating cells involved in cardiac disease, even in the acute stage.
AcknowledgEment
The authors would like to thank Dr Hongjian Shi for assistance with MRI scanning.
REFERENCES
