Lars Alexander Schneider
University Department of Dermatology & Allergology Ulm, University Department of Immunology Ulm, Maienweg 12, D-89081 Ulm, Germany. E-mail: lars-alexander.schneider@imail.de
Accepted April 20, 2006.
Sir,
Primary cutaneous B-cell lymphoma (CBCL) is rare and treatment standards are therefore not based on large clinical studies. A modern therapeutic approach is rituximab, a monoclonal anti-CD20 antibody. We describe here a patient that demonstrates the need for close histological follow-up, when using this antibody therapy, because of the high rate of relapses.
Case Report
A 69-year-old woman presented with a 2-year history of asymptomatic violaceous nodules, plaques and tumours on her left forehead and scalp (Fig. 1a) and was diagnosed with a multifocal, primary cutaneous follicular centre cell lymphoma (2005 WHO-EORTC classification) (1–3).The initial histological picture is shown in Fig. 2a. No systemic symptoms were present and staging investigations (bone marrow histology, cytometry of peripheral blood cell fractions, abdominal ultrasound, computed tomography (CT) scan from skull to pelvis) excluded disease manifestation apart from the skin lesions. Immunohistochemistry revealed a diffuse infiltrate of larger lymphoid cells resembling centroblasts, together with smaller cells resembling centrocytes. The infiltrating cells were more than 90% CD20+, CD79a+ and BCL2-negative. In addition, some scattered CD4+ and CD8+ T-cells and CD21+ dendritic cells were detected.
Presented with the diagnosis and the options of radical excision or fractionated radiotherapy after surgical mapping of the area, the patient did not want to undergo either of the two treatment options, for fear of severe disfigurement. We therefore decided to treat her with rituximab as front-line therapy in this case. Because of the diffuse character of the clinical presentation, systemic administration according to the scheme published by Gellrich et al. (4) was chosen rather than intra-lesional rituximab. A total of 8 weekly courses of intravenous rituximab, 375mg/m2 body surface area, were given and complete clinical remission of the CBCL was observed, as shown in Fig 1b.
To confirm the outcome, we took a new punch biopsy from an area of complete clinical remission 2 months after the last rituximab (Mabthera®, Roche, Basel, Switzerland) infusion and found cutaneous CBCL-residues of the same immunophenotype as before (Fig. 2b). Further mapping-biopsies were then taken to localize the area with residues more precisely, and clinically non-apparent lymphoma patches were also found at other sites. Therefore the patient underwent an additional course of fractionated soft-tissue radiotherapy (in total 40 Gray). Following radiotherapy the patient has been in histological controlled complete remission for over one year.
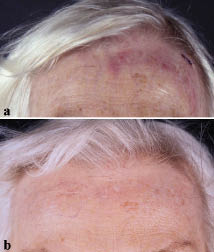
Fig. 1. (a) Initial clinical presentation with violaceous, diffuse and asymptomatic nodules on the forehead, and (b) 2 months after 8 courses of rituximab, complete clinical remission of the lymphoma is seen.
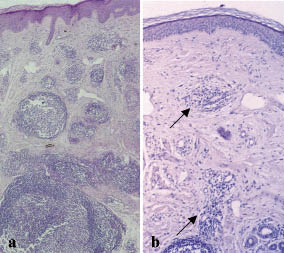
Fig. 2. (a) Initial histology with dense nodular lymphocytic infiltrates forming lymphoid follicular structures throughout dermis and subcutaneous layer, and (b) control biopsy taken at the same time as Fig. 1(b). Residual focal follicular infiltrations of lymphocytes can be seen in the dermis (arrows), less dense, however, than at initial presentation.
Discussion
With more than 30 publications on the subject in the last 6 years, systemic and intra-lesional rituximab (monoclonal chimaeric anti-CD20 antibody) has gained much attention as a therapy for CD20-positive primary CBCL (4–12). CBCL as such is rare and represents an entity to be distinguished from nodal B-cell non-Hodgkin’s-lymphoma with secondary skin involvement (13) because of restriction to the skin, long-term survival and high incidence of local recurrence after various treatment modalities. The clinical course, apart from a subgroup, the “large cell lymphoma of the lower limb” is generally favourable, with consistently reported follow- up 5-year survival rates over 80% (13–17).
However, one needs to be sure that the staging investigations are performed with accuracy in order not to miss secondary systemic involvement. In our case this was done via histological and cyto-morphological assessment of peripheral blood and bone marrow alongside imaging with CT scans to exclude tumour involvement of the lymphoid organs. Recently, one publication has pointed to a new and possibly more advanced strategy using 18-FDG positron emission tomography, which allows the physiological tracking of proliferating B-cells (18). This strategy might offer some advantages in the future.
In the absence of controlled trials, there is still no clear consensus about the treatment modality of choice in CBCL. Several approaches have been effective (13, 16, 19), and thus the clinical management decisions for therapy and follow-up are frequently made based on the findings in individual cases. This can be difficult in cases presenting with multifocal diffuse and deep cutaneous infiltrates, such as presented here, when margins for excision or radiotherapy are difficult to define, even with prior surgical tumour mapping.
In this setting rituximab appears to be a reasonable treatment option as it offers the potential advantage of a systemic mode of action, when given intravenously. The clinical treatment success we observed supported this view and was encouraging, however, and this is the important point in our view: clinical remission alone is not a sufficient criterion of therapeutic success.
Reviewing the literature, it is known that in more than 50% of cases reported (6–11, 20), no complete remission is achieved with rituximab alone and this fact makes routine histological control biopsies mandatory in all patients treated with this antibody.
References
1. Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005; 105: 3768–3785.
2. Hoefnagel JJ, Dijkman R, Basso K, Jansen PM, Hallermann C, Willemze R, et al. Distinct types of primary cutaneous large B-cell lymphoma identified by gene expression profiling. Blood 2005; 105: 3671–3678.
3. Willemze R, Kerl H, Sterry W, Berti E, Cerroni L, Chimenti S, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood 1997; 90: 354–371.
4. Gellrich S, Muche JM, Wilks A, Jasch KC, Voit C, Fischer T, et al. Systemic eight-cycle anti-CD20 monoclonal antibody (rituximab) therapy in primary cutaneous B-cell lymphomas – an applicational observation. Br J Dermatol 2005; 153: 167–173.
5. Roguedas AM, Watier H, Paintaud G, de Muret A, Vaillant L, Machet L. Intralesional therapy with anti-CD20 monoclonal antibody rituximab: local and systemic efficacy in primary cutaneous B-cell lymphoma. Br J Dermatol 2005; 152: 541–544.
6. Aboulafia DM. Primary cutaneous large B-cell lymphoma of the legs: a distinct clinical pathologic entity treated with CD20 monoclonal antibody (rituximab). Am J Clin Oncol 2001; 24: 237–240.
7. Paul T, Radny P, Krober SM, Paul A, Blaheta HJ, Garbe C. Intralesional rituximab for cutaneous B-cell lymphoma. Br J Dermatol 2001; 144: 1239–1243.
8. Soda R, Costanzo A, Cantonetti M, Orlandi A, Bianchi L, Chimenti S. Systemic therapy of primary cutaneous B-cell lymphoma, marginal zone type, with rituximab, a chimeric anti-CD20 monoclonal antibody. Acta Derm Venereol 2001; 81: 207–208.
9. Gellrich S, Muche JM, Pelzer K, Audring H, Sterry W. Der Anti-CD20-Antikörper bei primär kutanen B-Zell-Lymphomen Erste Erfahrungen in der dermatologischen Anwendung. Hautarzt 2001; 52: 205–210.
10. Heinzerling L, Dummer R, Kempf W, Schmid MH, Burg G. Intralesional therapy with anti-CD20 monoclonal antibody rituximab in primary cutaneous B-cell lymphoma. Arch Dermatol 2000; 136: 374–378.
11. Sabroe RA, Child FJ, Woolford AJ, Spittle MF, Russell-Jones R. Rituximab in cutaneous B-cell lymphoma: a report of two cases. Br J Dermatol 2000; 143: 157–161.
12. Lacouture ME, Baron JM, Jani AB, Laumann AE, Soltani K. Treatment of radiation-relapsing primary cutaneous B-cell lymphoma with an anti-CD20 monoclonal antibody. Clin Exp Dermatol 2005; 30: 46–48.
13. Santucci M, Pimpinelli N, Arganini L. Primary cutaneous B-cell lymphoma: a unique type of low-grade lymphoma. Clinicopathologic and immunologic study of 83 cases. Cancer 1991; 67: 2311–2326.
14. Kerl H, Volkenandt M, Cerroni L. [Malignant lymphoma of the skin]. Hautarzt 1994; 45: 421–443.
15. Pimpinelli N, Santucci M, Mori M, Vallecchi C, Giannotti B. Primary cutaneous B-cell lymphoma: a clinically homogeneous entity? J Am Acad Dermatol 1997; 37: 1012–1016.
16. Rijlaarsdam JU, Toonstra J, Meijer OW, Noordijk EM, Willemze R. Treatment of primary cutaneous B-cell lymphomas of follicle center cell origin: a clinical follow-up study of 55 patients treated with radiotherapy or polychemotherapy. J Clin Oncol 1996; 14: 549–555.
17. Joly P, Vasseur E, Esteve E, Leibowitch M, Tilly H, Vaillant L, et al. Primary cutaneous medium and large cell lymphomas other than mycosis fungoides. An immunohistological and follow-up study on 54 cases. French Study Group for Cutaneous Lymphomas. Br J Dermatol 1995; 132: 506–512.
18. Shapiro M, Yun M, Junkins-Hopkins JM, Vittorio CC, Schulman N, Saidman BH, et al. Assessment of tumor burden and treatment response by 18F-fluorodeoxyglucose injection and positron emission tomography in patients with cutaneous T- and B-cell lymphomas. J Am Acad Dermatol 2002; 47: 623–628.
19. Peris K, Caracciolo E, Weirich G, Chimenti S. Primary centroblastic/centrocytic lymphoma of the skin. Detection of B-cell monoclonality by polymerase chain reaction. Am J Dermatopathol 1995; 17: 506–510.
20. Peker S, Neuber K, Hoeger PH, Hoelscher D, Bleck O, Moll I. Therapie eines kutanen B-Zell Lymphoms mit dem CD20 Antikörper Rituximab (MabThera®). Hautarzt 1999; 50: 71.
