OBJECTIVE: To assess the occurrence and risk factors for complications following spinal cord injury during and after inpatient rehabilitation.
DESIGN: Multicentre longitudinal study.
SUBJECTS: A total of 212 persons with a spinal cord injury admitted to specialized rehabilitation centres.
METHODS: Assessments at the start of active rehabilitation (n = 212), 3 months later (n = 143), at discharge (n = 191) and 1 year after discharge (n = 143).
RESULTS: Multi-level random coefficient analyses revealed that complications were common following spinal cord injury. Most subjects reported neurogenic and musculoskeletal pain, or had spasticity at each assessment. During the year after discharge, complications remained common: urinary tract infections and pressure sores affected 49% and 36% of the population, respectively. The degree of pain decreased, whereas the degree of spasticity increased significantly during inpatient rehabilitation. Overall, increased age, increased body mass index, traumatic lesion, tetraplegia, and complete lesion all increased the risk of complications.
CONCLUSION: Complications are common following spinal cord injury. They need specific attention after discharge from inpatient rehabilitation and within subpopulations.
Key words: spinal cord injury, complications, risk factors, rehabilitation.
J Rehabil Med 2007; 39: 393–398
Correspondence address: Janneke A. Haisma, Department of Rehabilitation Medicine, Erasmus MC, University Medical Center Rotterdam, PO Box 2040, 3000 CA Rotterdam, The Netherlands. E-mail: j.haisma@erasmusmc.nl
Submitted July 3, 2006; accepted January 12, 2007
Introduction
Spinal cord injury (SCI) is often followed by complications, which add to the detrimental effect that loss of motor, sensory and autonomic function have on a person’s health, social participation and quality of life (1–4). There is some debate about the operational definition of complications, about whether complications are strictly causally related to the SCI, and on how to distinguish complications from co-morbidity (5). However, a condition may only be defined as being a complication if it has a chronological relation with the SCI, i.e. the SCI needs to precede the condition. Additionally, although this condition may also occur in the general population, one assumes those with SCI to be at increased risk (5).
The range of conditions following SCI can be categorized into neurological consequences and secondary complications. Neurological consequences result from the injury itself, following interruption and decentralization of the nervous system, and may be regarded as sequelae to the injury (6). Examples are neurogenic pain or spasticity; the latter being part of the upper motor neurone syndrome (7, 8). Secondary complications follow the ensuing loss of pulmonary function, loss of bladder control or reduced mobility. Examples are pulmonary infections and pressure sores (1, 9, 10). Neurological consequences and secondary complications behave similarly; they often require, and react to, treatment, and they affect the rehabilitation process. Therefore, we refer to them all as complications of SCI.
Complications have a considerable impact on those with SCI. A high incidence of complications is associated with a lower level of health-related aspects, such as physical capacity, activities and functional outcome (1, 3, 4, 11). Complications may interfere with the start of active rehabilitation, can form a disappointing set-back during rehabilitation, and frequently lead to re-hospitalization (7, 12, 13). Additionally, complications are an important cause of mortality following SCI (5, 14). In order to optimize the individual rehabilitation process and outcome, it is important to predict and prevent complications or to recognize and treat them (5).
Previous studies have investigated complications following SCI and their risk factors. They have illustrated the association between subject and lesion characteristics and the occurrence of complications (1, 8, 15, 16). They have also showed that the diversity and occurrence of complications change over time (1, 9, 10, 17). However, these studies have limitations. Firstly, most studies were cross-sectional in design, whereas a longitudinal study in which data are collected prospectively could better establish which factors actually lead to complications. Secondly, in most studies only one or a few complications were assessed, whereas the simultaneous investigation of a range of complications will give insight into their diversity, and into how their occurrence compares. Therefore, we have formulated the following 3 research questions:
• What is the occurrence of complications (pain, spasticity, hypotension, autonomic dysreflexia, pressure sores, urinary tract infections, pulmonary infections, venous thromboembolism, oedema, heterotopic ossification, other cardiovascular disease and other musculoskeletal complaints) in subjects with SCI during and after inpatient rehabilitation?
• What is the degree of pain and spasticity and does this change during and after inpatient rehabilitation?
• What are the risk factors for these complications?
MethodS
The present study was part of the Dutch research program on the restoration of mobility in persons with SCI. Subjects admitted to one of the 8 participating rehabilitation centres between May 2000 and September 2003 were included if they met the eligibility criteria. During their first period of inpatient rehabilitation, they were eligible for inclusion if they were between 18 and 65 years of age, were wheelchair-dependent, had sufficient comprehension of the Dutch language to understand the purpose of the study, and did not have a progressive or psychiatric condition that would interfere with constructive participation.
Design
Subjects were assessed according to a standardized protocol at 4 assessment times: at the start of active inpatient rehabilitation, defined as the moment when the subject was able to sit in a wheelchair for ≥ 3 hours (t1), 3 months later (t2), at discharge (t3), and one year after discharge from inpatient rehabilitation (t4). If the subject was discharged within one month after t2, the assessment at t2 was considered a "discharge" assessment, and was included in the analyses of t3.
The protocol was approved by the Medical Ethics Committee and prior to participation all subjects gave their written informed consent.
Complications
Based on the subject’s history and medical chart, the physicians registered each complication on a standardized list. At t1, complications then present or that had occurred since admission to rehabilitation were registered. At t2 and t3, complications then present or that had occurred since t1 and t2, respectively, were registered. At t4, complications then present or that had occurred since discharge were registered. The occurrence of a complication was registered as follows: 0 = no complication; 1 = presence or history of this complication. Other cardiovascular disease and musculoskeletal complaints were grouped, and included conditions such as myocardial disease and bursitis occurring after the lesion, respectively.
If the subject reported having pain, the research assistants completed a standardized list addressing the nature of the pain. Musculoskeletal pain was defined as nociceptive pain originating from bone, joint or muscle structures following trauma or overuse (7). Thirteen locations (on the upper and lower limbs, the neck and the back) were assigned severity scores with a 5-point Likert scale (1–5: ranging from “not severe” to “very severe”), and frequency scores (1–3: ranging from “once a week or less” to “more than 3 times a week”). Neurogenic pain was defined as at-level or below-level pain originating from spinal cord ischaemia or trauma (7). Hence, 9 neurogenic pain characteristics (other pain, numbness, itching, tingling, cold, warm, perspiration, girdle zone pain and phantom feeling) were assigned frequency and severity scores. A sum score was made for the product (severity × frequency) of these locations or pain characteristics. Therefore, sum scores ranging from 1 to 195, or from 1 to 135 could be attained for musculoskeletal or neurogenic pain, respectively.
Additionally, the research assistant determined the presence of spasticity, defined as the velocity dependent increase in muscle tone combined with exaggerated reflexes, through a direct standardized examination (7). The left and right hip adductors, knee flexors and extensors, ankle extensors, and elbow flexors and extensors were examined. In the presence of spasticity, each muscle group was assigned severity scores ranging from 1 to 3 (1: catch; 2: clonus < 5 beats; 3: clonus ≥ 5 beats). These scores were summated, which gave a sum score ranging from 1 to 36.
Risk factors
The associations with the following potential risk factors were assessed: age, gender, smoking status (smoker vs non-smoker), body mass index (BMI: body mass in kilograms divided by height in metres squared; kg/m2), the cause (traumatic vs non-traumatic), the level and the completeness of the lesion. Although some smokers refrained from smoking during inpatient rehabilitation, nearly all resumed smoking after discharge. Therefore, subjects were defined as being a smoker if they smoked prior to the injury. Tetraplegia was defined as a lesion at or above the first thoracic segment, and paraplegia as a lesion below the first thoracic segment. A complete lesion was diagnosed in the absence of sensory or motor function in the sacral segments, i.e. American Spinal Injury Association (ASIA) category A. An incomplete lesion was defined as ASIA categories B, C or D (18).
Statistics
Random coefficient analyses (MlwiN version 1.1; Centre for Multilevel Modelling, Institute of Education, London, UK) were used to estimate the occurrence of complications, the change in the degree of pain and spasticity, and the association with risk factors (19, 20). The longitudinal aspect of the study is enhanced by the fact that the analyses can be done with missing values and varying group composition. Therefore, all assessments can be included in the analyses, which gives a more accurate description, compared with repeated measurements analysis of variance, for example, of complications and their risk factors at each assessment.
Analysis of complications. A logistic random coefficient model was made for the occurrence of each complication. Time was included in the model as a set of 3 dummy variables (each with their own regression coefficient). The discharge assessment was chosen as their reference and was estimated by the intercept. The occurrence of a complication during the other intervals was estimated as follows: 1/ {1 + exp[ – (intercept + regression coefficient)]} (19). The sum scores for pain and spasticity were estimated with similar models for continuous outcome variables. Again, time was modelled as 3 dummy variables, and the score at discharge was estimated by the intercept. The sum scores at the other assessments were calculated by adding the intercept to the regression coefficient of the dummy variable.
Analysis of risk factors. All risk factors were simultaneously added to the previously described models. With these multivariate models, the contribution of each risk factor was corrected for. The regression coefficients for the risk factors were converted to odds ratios (ORs); OR = exp[regression coefficient]. An OR of 1 indicated that there was no association with this particular variable, whereas an OR < 1 indicated a decreased risk, and an OR > 1 indicated an increased risk of this complication in the presence of the risk factor. For the continuous outcome variables, the regression coefficient indicated the difference in the sum score associated with the difference in the risk factor of 1 unit.
Results
Table I gives the descriptive characteristics of the subjects. Subjects were lost to follow-up for several reasons: 9 died, 5 moved abroad, 26 refused further participation, 7 developed a psychiatric or progressive condition, 5 were untraceable and 3 dropped out for unknown reasons. Forty-four subjects were discharged within one month after t2, and therefore, no data were included for them at t2. The mean (SD) duration between injury and admission to rehabilitation was 44 (43) days.
| Table I. Descriptive subject characteristics. |
| | Start | 3 months | Discharge | 1 year after discharge |
| Subjects (n) | 212 | 143 | 191 | 143 |
| Age (years) mean (SD) | 40 (14) | 41 (14) | 41 (14) | 41 (14) |
| Gender (% male) | 74 | 75 | 73 | 73 |
| Body mass index (kg/m2) mean (SD) | 22.7 (3.8) | 23.1 (4.0) | 23.4 (4.0) | 24.5 (4.5) |
| Smoker (% smoker) | 44 | 44 | 45 | 48 |
| Days since previous assessment* mean (SD) | 49 (44) | 103 (33) | 162 (125) | 397 (57) |
| Cause (% traumatic) | 74 | 78 | 75 | 76 |
| Level (% tetraplegia) | 41 | 48 | 40 | 35 |
| Completeness (% complete) | 45 | 44 | 49 | 50 |
| *For the start of active rehabilitation, days since admission are given. |
Complications
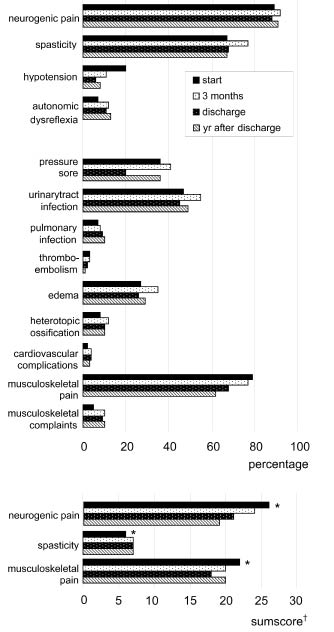
Fig. 1 shows the estimated occurrence of a complication during each interval. All data are derived from random coefficient analyses. Most subjects reported neurogenic and musculoskeletal pain, or had spasticity at each assessment. Common secondary complications were urinary tract infections and pressure sores, reported by 47% and 36% of the population at t1, respectively. Like most complications, they remained common during the year after discharge, occurring in 49% and 36% of the population at t4, respectively. Additionally, Fig. 1 shows the estimated degree of pain and spasticity at each assessment. The degree of pain decreased, whereas the degree of spasticity increased significantly during inpatient rehabilitation.
Fig. 1. The estimated occurrence of a complication during each interval and the sum scores for pain or spasticity at 4 assessment times: random coefficient modelling. *Significant difference (p ≤ 0.05) between the sum score at t1 and that at t3. †Sum scores determined in those subjects who reported pain or who had spasticity at examination, expressed as a percentage of the maximum obtainable score.
Risk factors
Table II gives the ORs for the association between the risk factors and the occurrence of a complication. An increase in age, tetraplegia and completeness of the lesion were the most frequently identified risk factors. Those with a high BMI and those with a traumatic lesion were at increased risk of several complications; the largest effect was seen for cardiovascular disease. The risk associated with gender or smoking varied for different complications.
| Table II. Odds ratios for the association with potential risk factors: multivariate logistic random coefficient modelling. |
| | Age (years) | Gender* | Smoking* | Body mass index (kg/m2) | Cause* | Level* | Completeness* |
| Neurogenic pain | 1.02 (0.99; 1.05) | 0.52 (0.23; 1.21) | 1.20 (0.63; 2.27) | 0.99 (0.91; 1.08) | 0.99 (0.41; 2.41) | 0.86 (0.44; 1.70) | 0.57 (0.29; 1.11) |
| Spasticity | 0.97 (0.96; 0.99) | 2.53 (1.60; 3.98) | 1.42 (0.93; 2.16) | 1.11 (1.04; 1.18) | 1.07 (0.64; 1.79) | 0.13 (0.08; 0.23) | 0.95 (0.61; 1.48) |
| Hypotension | 0.99 (0.97; 1.02) | 0.82 (0.43; 1.54) | 0.73 (0.41; 1.29) | 0.97 (0.90; 1.04) | 1.38 (0.59; 3.25) | 0.09 (0.05; 0.18) | 2.44 (1.32; 4.52) |
| Autonomic dysreflexia | 0.98 (0.95; 1.00) | 1.69 (0.81; 3.52) | 1.21 (0.68; 2.15) | 1.07 (0.99; 1.15) | 0.94 (0.40; 2.21) | 0.14 (0.07; 0.27) | 2.36 (1.26; 4.42) |
| Pressure sore | 1.01 (1.00; 1.03) | 1.08 (0.72; 1.63) | 1.34 (0.93; 1.93) | 0.98 (0.93; 1.03) | 1.08 (0.67; 1.74) | 0.53 (0.36; 0.78) | 1.73 (1.17; 2.56) |
| Urinary tract infection | 1.00 (0.99; 1.02) | 0.87 (0.60; 1.28) | 0.78 (0.56; 1.11) | 0.97 (0.93; 1.02) | 1.59 (1.02; 2.47) | 0.52 (0.36; 0.75) | 1.81 (1.26; 2.60) |
| Pulmonary infection† | 1.05 (1.02; 1.08) | 1.03 (0.51; 2.07) | 1.49 (0.79; 2.80) | 0.93 (0.85; 1.01) | 1.04 (0.45; 2.42) | 0.26 (0.13; 0.53) | 3.53 (1.74; 7.17) |
| Thromboembolism | 1.01 (0.97; 1.06) | 0.69 (0.22; 2.12) | 2.10 (0.72; 6.11) | 0.92 (0.79; 1.08) | – ‡ | 1.35 (0.42; 4.32) | 1.84 (0.59; 5.74) |
| Oedema | 1.04 (1.03; 1.06) | 0.81 (0.53; 1.24) | 1.62 (1.09; 2.41) | 1.10 (1.04; 1.16) | 2.05 (1.23; 3.43) | 1.09 (0.72; 1.65) | 1.46 (0.97; 2.21) |
| Heterotopic ossification | 0.98 (0.96; 1.01) | 11.38 (2.74; 47.32) | 0.50 (0.27; 0.91) | 1.01 (0.93; 1.09) | 0.79 (0.35; 1.78) | 0.80 (0.42; 1.49) | 2.45 (1.29; 4.67) |
| Cardiovascular disease | 1.05 (1.01; 1.10) | 0.71 (0.24; 2.13) | 1.28 (0.41; 3.96) | 1.28 (1.11; 1.46) | 5.06 (1.25; 20.46) | 0.90 (0.31; 2.63) | 0.60 (0.19; 1.94) |
| Musculoskeletal pain | 1.00 (0.98; 1.02) | 0.63 (0.40; 0.99) | 0.78 (0.53; 1.14) | 1.07 (1.01; 1.13) | 1.92 (1.17; 3.15) | 0.66 (0.43; 1.00) | 0.73 (0.48; 1.09) |
| Other musculoskeletal complaints | 1.02 (1.00; 1.05) | 1.11 (0.56; 2.19) | 1.06 (0.58; 1.93) | 0.96 (0.89; 1.04) | 1.33 (0.60; 2.96) | 0.86 (0.45; 1.64) | 1.53 (0.81; 2.89) |
| Odds ratios (and 95% confidence intervals) are given; significant associations (p ≤ 0.05) are printed in bold. *Gender: men = 1, women = 0; Smoking: smoker = 1, non-smoker = 0; Cause: traumatic = 1, non-traumatic = 0; Level: paraplegia = 1, tetraplegia = 0; Completeness: complete = 1, incomplete = 0. †As an example is given that an increase in age of one year was associated with a 1.05 times larger risk of a pulmonary infection; those with a complete lesion were 3.53 times more at risk of a pulmonary infection, than those with an incomplete lesion. ‡ “Cause” could not be modelled; in this population all subjects with thromboembolism had a traumatic lesion. |
Table III gives the regression coefficients for the association between the degree of pain or spasticity and the risk factors. Overall, an increase in age was associated with an increase in pain. Men and those with a traumatic lesion had a higher degree of spasticity. Those with a tetraplegia and those with an incomplete lesion reported more musculoskeletal pain and showed a higher degree of spasticity.
| Table III. Regression coefficients for the association between risk factors and the degree of pain or spasticity: multivariate random coefficient modelling. |
| | Age | Gender* | Smoking* | Body mass index (kg/m2) | Cause* | Level* | Completeness* |
| Neurogenic pain | 0.12 (0.01; 0.23) | –2.20 (–5.30; 0.89) | –2.29 (–5.10; 0.52) | 0.27 (–0.11; 0.64) | –1.28 (–4.86; 2.30) | –9.84 (–12.81; –6.88) | 2.86 (–0.10; 5.82) |
| Spasticity | 0.00 (–0.03; 0.03) | 1.02 (0.13; 1.90) | 0.64 (–0.11; 1.38) | 0.02 (–0.09; 0.12) | 2.33 (1.35; 3.32) | –1.07 (–1.86; –0.28) | –2.21 (–3.02; –1.39) |
| Musculoskeletal pain† | 0.14 (0.01; 0.28) | –2.33 (–6.08; 1.43) | –2.13 (–5.58; 1.32) | 0.06 (–0.39; 0.50) | –1.24 (–5.76; 3.27) | –10.03 (–13.61; –6.44) | –4.60 (–8.26; –0.94) |
| Regression coefficients (and 95% confidence intervals) are given; significant associations (p ≤ 0.05) are printed in bold. *Gender: men = 1, women = 0; Smoking: smoker = 1, non-smoker = 0; Cause: traumatic = 1, non-traumatic = 0; Level: paraplegia = 1, tetraplegia = 0; Completeness: complete = 1, incomplete = 0. † As an example is given that the sum score for musculoskeletal pain was 10.03 points lower for those with paraplegia than for those with tetraplegia. |
Discussion
Complications
The present study showed that the occurrence of a range of complications was high both during and after inpatient rehabilitation. Most of our findings coincided with previous studies, but inconsistencies may be attributed to the variation in both the selected population and the design. Excluding those with progressive disease or those over the age of 65 years, for example, will have influenced the findings. Complications were registered based on clinical symptoms often confirmed by additional examination, whereas some others searched for pathology in the absence of clinical symptoms. Furthermore, the longitudinal design will have revealed different information on the association with risk factors than did previous cross-sectional data.
Although pain and spasticity were common, understanding their impact remains challenging. The reported degree of pain is influenced by both psychosocial and physical factors, which interfere with the interpretation of changes over time and the associations with risk factors (21, 22). It remains to say, that when pain is experienced, it does often necessitate intervention. Besides increased stretch reflexes, spasticity encompasses increased muscle tone, involuntary movements and primitive reflexes. Lack of effective measurement techniques makes the quantification of all components of spasticity difficult (7). Furthermore, in contrast to pain, the degree of spasticity is not necessarily related to individual functional complaints or to the required treatment. Because pain and spasticity are common sequelae to SCI, which may require intervention, it is important to reach consensus on how to monitor their clinical impact.
Several mechanisms may explain why complications are common after discharge from inpatient rehabilitation (9, 10, 12). After discharge, both the demanding activities of daily living (ADLs) and the reduction in structured training, could make subjects susceptible to complications associated with overuse (1). Additionally, many subjects have less guidance or peer-control in self-care (23). The higher demands of ADLs, together with reduced feedback, could make subjects less conscientious towards preventive measures, such as skin checks and bladder management. The active involvement of the spouse may contribute to the prevention of complications, and further analyses of our data showed a tendency for those living alone to be at increased risk. Although the interval evaluated after discharge is long compared with the intervals during rehabilitation, complications remain a common problem. This should warrant decision-makers investing in effective follow-up programs (3, 12, 23). Persons with SCI have an increasing life expectancy, and it is important for rehabilitation medicine to strive for the long-term prevention of complications.
Risk factors
This study indicated subpopulations inherently at risk of complications. The level and completeness of the lesion determine the extent of pareses and loss of respiratory and autonomic function (1, 24, 25), which are inevitably associated with the risk of complications (1, 10, 22). The physiological age-related decline in cardio-respiratory function and mobility leave the elderly susceptible to complications (9, 26). Concurrent extra-spinal injury may be an additional risk factor (27). Although concurrent injury was poorly registered in the present study, further investigation revealed that it was more common in traumatic lesions. This may partly explain why those with a traumatic lesion were at risk of some complications. However, a standardized registration of concurrent injury is needed to establish these correlations. The unexplained predisposition of men for heterotropic ossification has been described before (13). It could be that in men symptoms were more apparent, or more often attributed to heterotopic ossification. Although type of lesion, age and gender cannot be influenced, their identification as important risk factors allows us to target screening and prevention programs.
Besides these unchangeable risk factors, the effect of some lifestyle risks was investigated. An increase in BMI increased the risk of several complications. However, the interpretation of the BMI is difficult in those with SCI, especially during the early phase of rehabilitation (28). Changes in body weight may be attributed to an increase in upper body muscle mass, a decrease in muscle mass of the lower limbs, the accumulation of extra-vascular fluids and an increase in fat mass (28). The associations with other modifiable lifestyle risks, such as alcohol consumption and inactivity, remain ambiguous (29, 30). We anticipate that the cumulative damaging effect of smoking, alcohol consumption, dietary intake and inactivity will become more evident at a later phase post-injury. Therefore, the correlation between lifestyle exposure and complications needs to be investigated in a longitudinal study with a longer follow-up period.
Limitations
The present study design needs some consideration. One should bear in mind that the study investigated medical complications, whereas SCI also has psychosocial complications beyond the scope of this investigation. In general, data on the occurrence of complications during a time interval do not inform us about their severity or duration. Because the occurrence of a complication may have delayed discharge, the population assessed 3 months into active inpatient rehabilitation was probably more prone to complications. The assessed population and the varying intervals should be considered when interpreting results. However, random coefficient analyses has allowed us to include all present subjects at each assessment point and this has provided more realistic data on the occurrence of complications during each interval. Our longitudinal design provided insight into the timing of complications, and contributed to the understanding of the nature of risk factors during different phases of rehabilitation.
In conclusion, complications are common following SCI and subpopulations are at increased risk. Educational programs for patients and their families need to focus on the prevention and early recognition of complications. Structural follow-up visits after inpatient rehabilitation need to be implemented at specialized rehabilitation centres. Besides addressing functional outcome and social participation, these visits also need to focus on complications. Only through the timely prevention, surveillance and treatment of complications can their impact be reduced, and can the individual rehabilitation outcome be optimized.
Acknowledgements
This article was presented in part at the 8th International Neurotrauma Symposium, 21–25 May 2006, Rotterdam, The Netherlands, and at the 45th ISCoS Annual Scientific Meeting, 25–28 June 2006, Boston, USA.
This study was supported by the Health Research and Development Council of The Netherlands (grants 1435.0003; 1435.0025).
References
1. Noreau L, Proulx P, Gagnon L, Drolet M, Laramee MT. Secondary impairments after spinal cord injury: a population-based study. Am J Phys Med Rehabil 2000; 79: 526–535.
2. Westgren N, Levi R. Quality of life and traumatic spinal cord injury. Arch Phys Med Rehabil 1998; 79: 1433–1439.
3. Bloemen-Vrencken JH, Post MW, Hendriks JM, De Reus EC, De Witte LP. Health problems of persons with spinal cord injury living in the Netherlands. Disabil Rehabil 2005; 27: 1381–1389.
4. Valtonen K, Karlsson AK, Alaranta H, Viikari-Juntura E. Work participation among persons with traumatic spinal cord injury and meningomyelocele1. J Rehabil Med 2006; 38: 192–200.
5. Meyers AR, Andresen EM, Hagglund KJ. A model of outcomes research: spinal cord injury. Arch Phys Med Rehabil 2000; 81: S81–S90.
6. Kirschblum S, Campagnolo DI, DeLisa JA, editors. Spinal cord medicine. Philadelphia: Lippincott Williams & Wilkins; 2002.
7. Burchiel KJ, Hsu FP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine 2001; 26: S146–S160.
8. Werhagen L, Budh CN, Hultling C, Molander C. Neuropathic pain after traumatic spinal cord injury – relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord 2004; 42: 665–673.
9. McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil 1999; 80: 1402–1410.
10. Chen Y, Devivo MJ, Jackson AB. Pressure ulcer prevalence in people with spinal cord injury: age-period-duration effects. Arch Phys Med Rehabil 2005; 86: 1208–1213.
11. Post MW, Dallmeijer AJ, Angenot EL, van Asbeck FW, van der Woude LH. Duration and functional outcome of spinal cord injury rehabilitation in the Netherlands. J Rehabil Res Dev 2005; 42: 75–85.
12. Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil 2004; 85: 1757–1763.
13. Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med 2005; 37: 129–136.
14. DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil 1999; 80: 1411–1419.
15. Aito S. Complications during the acute phase of traumatic spinal cord lesions. Spinal Cord 2003; 41: 629–635.
16. New P, Rawicki HB, Bailey MJ. Nontraumatic spinal cord injury rehabilitation: pressure ulcer pattern, prediction and impact. Arch Phys Med Rehabil 2004; 85: 87–93.
17. Chen D, Apple DF Jr., Hudson LM, Bode R. Medical complications during acute rehabilitation following spinal cord injury – current experience of the model systems. Arch Phys Med Rehabil 1999; 80: 1397–1401.
18. Maynard FM Jr., Bracken MB, Creasey G, Ditunno JF Jr., Donovan WH, Ducker TB, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274.
19. Twisk JW. Applied longitudinal data analysis for epidemiology; a practical guide. Cambridge: Cambridge University Press; 2003.
20. Rasbash J, Browne W, Goldstein H, Yang M, Plewis I, Healy M, et al. A user’s guide to MLwiN. London: University of London: 2002.
21. Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord 2001; 39: 63–73.
22. van Drongelen S, de Groot S, Veeger HE, Angenot EL, Dallmeijer AJ, Post MW, et al. Upper extremity musculoskeletal pain during and after rehabilitation in wheelchair-using persons with a spinal cord injury. Spinal Cord 2006; 44: 152–159.
23. Bloemen-Vrencken JH, de Witte LP. Post-discharge nursing problems of spinal cord injured patients: on which fields can nurses contribute to rehabilitation? Clin Rehabil 2003; 17: 890–898.
24. Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 2003; 82: 803–814.
25. Haisma JA, Bussmann JB, Stam HJ, Sluis TA, Bergen MP, Dallmeijer AJ, et al. Changes in physical capacity during and after inpatient rehabilitation in subjects with a spinal cord injury. Arch Phys Med Rehabil 2006; 87: 741–748.
26. DeVivo MJ, Kartus PL, Rutt RD, Stover SL, Fine PR. The influence of age at time of spinal cord injury on rehabilitation outcome. Arch Neurol 1990; 47: 687–691.
27. van Asbeck FW, Post MW, Pangalila RF. An epidemiological description of spinal cord injuries in The Netherlands in 1994. Spinal Cord 2000; 38: 420–424.
28. Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord 2005; 43: 513–518.
29. Janssen TW, van Oers CA, van Kamp GJ, TenVoorde BJ, van der Woude LH, Hollander AP. Coronary heart disease risk indicators, aerobic power, and physical activity in men with spinal cord injuries. Arch Phys Med Rehabil 1997; 78: 697–705.
30. Tate DG, Forchheimer MB, Krause JS, Meade MA, Bombardier CH. Patterns of alcohol and substance use and abuse in persons with spinal cord injury: risk factors and correlates. Arch Phys Med Rehabil 2004; 85: 1837–1847.