Rehabilitation medicine is entering a new era, based on the knowledge that the central nervous system has a substantial capacity for repair and regeneration. This capacity is used in 3 distinct but overlapping situations: (i) routine housekeeping throughout life (i.e. taking care of normal wear-and-tear); (ii) older age, when functional reserves of various kinds are depleted, resulting in cognitive, motor, and other deficits; and (iii) contexts in which a neurological deficit reflects an acute or chronic pathological process, such as neurotrauma, stroke, or neurodegenerative disease. The positive message here is two-fold. First, some aspects of regeneration occur even in the adult and ageing brain and spinal cord, and we are starting to unravel the underlying molecular mechanisms. Secondly, novel therapeutic approaches and targets are emerging that will substantially increase the efficiency and efficacy of rehabilitation and will transform rehabilitation into a discipline focusing both on its traditional domain and on prevention, ultimately across all the age categories. This review attempts to sum up the present knowledge about an enriched environment, currently the single most efficient plasticity- and regeneration-promoting paradigm. It also summarizes research showing that astrocytes – considered only years ago merely to nurse and support neurones – are a novel and highly interesting target for regenerative strategies in the brain and spinal cord.
Key words: enriched environment, astrocytes, astrocyte intermediate filaments, plasticity, regeneration, stroke, rehabilitation, brain, stimulation.
J Rehabil Med 2007; 39: 345–352
Correspondence address: Michael Nilsson or Milos Pekny, Center for Brain Repair and Rehabilitation (CBR), Department of Clinical Neuroscience and Rehabilitation, Institute for Neuroscience and Physiology, Sahlgrenska Academy, Göteborg University, Guldhedsgatan 19, SE-413 45 Göteborg, Sweden. E-mail: michael.nilsson@vgregion.se,
E-mail: milos.pekny@medkem.gu.se
Submitted March 9, 2007; accepted April 13, 2007
*This paper is based partly on a lecture given at the international symposium ”Evidence for stroke rehabilitation – bridging into the future”, in Göteborg, Sweden, 26–28 April, 2006.
INTRODUCTION
Stroke is a major cause of death and the primary cause of adult disability in many countries (1). More than 60% of survivors suffer persistent neurological deficits (2) and need comprehensive rehabilitation. Recovery of central nervous system (CNS) function after injury, and stroke in particular, has long been a primary goal of neuroscience research. Understanding changes in the brain after an insult – and the extent to which they influence the potential for regeneration – is of fundamental importance in developing strategies for functional recovery. By promoting CNS regeneration, such strategies are expected to positively affect specific repair mechanisms involved in brain plasticity after injury or stroke.
In contrast to the recognized regenerative capacity of neurones in the peripheral nervous system (3, 4), the immature CNS, and the CNS of lower vertebrates (5), efforts to induce regeneration in the adult mammalian CNS have been disappointing. However, after stroke, neural progenitor cells in the subventricular zone, 1 of the 2 main neurogenic niches in the adult brain, can proliferate, migrate into the damaged area, and differentiate into neurones, replacing some, albeit very few, of the neuronal cells lost to ischaemia (6–8). This is a very encouraging message. Thus, regeneration research should focus on re-establishing disrupted and establish compensatory connections within the neuronal and astrocytic networks and on replacing cells lost to pathological processes.
It is becoming increasingly clear that many CNS elements contribute to degeneration and regeneration and that cells other than neurones are important players – and thus potential therapeutic targets – in these processes (9). The challenge is to harness cellular defence mechanisms and intervene before the environment becomes less conducive to regeneration and to continue applying therapeutic strategies thereafter. Understanding the cellular mechanisms of damage inflicted by cerebral ischaemia or traumatic brain injury is an essential step in meeting this challenge. Equally important is to determine the mechanisms that block regeneration. Both will form the basis for devising strategies to promote neural recovery.
ENRICHED ENVIRONMENT
Functional recovery after brain injury is dependent on the plasticity of both the cerebral cortex and unaffected parts of functional neuronal and astrocyte networks (10). Neural plasticity is an intrinsic property that enables the mammalian brain to adapt to environmental changes during development and adulthood. Plasticity is not static. It is an active, continuous process throughout life (11). By design, the brain is remarkably responsive to environmental stimuli, physiological modifications, and experiences (10), and its structure can be altered by experience in several measurable ways. In animals and humans, some regions in the normal adult brain, particularly the cortex, can alter their biochemistry, structure, and function – for example, during learning or in response to an enriched environment (EE) (12).
First described by Donald Hebb (13), the experimental paradigm of EE is the most widely used animal model of experience-induced plasticity. The term refers to an environment that provides greater possibilities for physical and social stimulation and/or interaction than standard housing conditions (14). EE is defined as “a combination of complex inanimate and social stimulation” (15), indicating that the interaction of several factors is the essential feature of the EE. As Hebb’s work and subsequent studies demonstrated, animals exposed to EE exhibit superior performance in several tests of higher-order cognitive ability (16, 17).
To maximize the effectiveness of rehabilitation therapies after stroke, it is critical to determine how the brain responds to different types of stimuli. Neural connections and cortical maps are continuously remodelled by our experience. Many studies have investigated the effects of EE on different aspects of brain plasticity in the intact brain and after brain injury, including stroke. Collectively, these studies show that EE has profound effects on behaviour, induces substantial structural and cellular changes, and specifically alters the levels of various neuroactive compounds (18–20). In animals, EE can induce pronounced biochemical, morphological and functional changes in uninjured as well as in injured or diseased brain. EE influences the uninjured brain in many ways that ultimately modulate its function. EE and other forms of complex stimulation increase brain weight and cortical and hippocampal thickness (21), the branching, length, and spine density of dendrites, and the size and number of discontinuous synapses (22–24). Furthermore, EE enhances neurogenesis in the hippocampus (25, 26). EE also increase levels of certain neurotrophic factors (e.g. brain-derived neurotrophic factor and nerve growth factor) that have important roles in neural signalling and cellular plasticity (27, 28).
Enriched environment as prevention
EE has also been shown partially to prevent or reduce the consequences of injuries or diseases in the CNS. With respect to EE and prevention, one of the most studied animal models is the R6/1 transgenic mouse, a model of Huntington’s disease (HD). In these mice, EE greatly reduces the onset of the motor symptoms (29) and delays the degenerative loss of cerebral volume (29). In a recent epidemiological study, environmental factors clearly modulated the clinical onset of HD (30). A similar effect has been observed in mouse models of Alzheimer’s disease (AD) (31) and in humans with AD (32). Indeed, EE may help to slow or prevent AD-associated cognitive decline (32, 33), a view supported by epidemiological studies (34). Interesting findings have also been presented on the effects of EE on onset and progression of disease symptoms in animal models of Parkinson’s disease and epilepsy (35, 36).
Enriched environment as a therapeutic modality
The therapeutic potential of EE has been evaluated in animal models of various neurological conditions, particularly stroke and traumatic brain injury. For example, EE enhances the recovery of motor function after focal brain ischaemia induced by middle cerebral artery occlusion (22, 37). It also has beneficial effects on cognitive functions such as memory and learning (38, 39). Exposure to EE after experimental brain trauma significantly improves both motor and cognitive functions (40, 41). In combination with multi-modal early-onset stimulation, EE reverses motor deficits in a model of brain trauma (42). These results suggest that EE combined with a “rehabilitation” program might act synergistically to enhance functional outcome. In the clinical setting, encouraging efforts have been made to provide an enriched and stimulating environment tailored to the needs of the patient and based on real-world experiences (43).
The morphological and biochemical correlates of EE- induced improvements are naturally manifold. For instance, in experimental models, EE reduces lesion size after brain trauma, is neuroprotective and increases dendritic outgrowth and the production of trophic factors (23, 27, 28, 36, 42). After experimental stroke, EE normalizes the astrocyte/neurone ratio in rats and seems to induce newly born progenitor cells to adopt a glial fate (44, 45).
Currently, EE is providing a novel therapeutic platform from which it is possible to derive molecular targets for the development of “enviromimetics” – new classes of pharmacological agents that would either mimic or enhance the positive effects of EE. In normal mice, exposure to EE alters the expression of genes involved in plasticity and neural signalling in different areas of the brain (46). In post-ischaemic rats housed under enriched conditions, we have observed large changes with a clear temporal profile in the expression of genes involved in cellular plasticity in the hippocampus (work in progress). Such approaches have the potential to guide further investigation into the function of EE-induced proteins and can ultimately be used in combination with modern neurorehabilitation strategies.
Studies of environmentally driven plasticity in the brain have traditionally focused on altered neuronal function, in particular synapse function. However, astrocytes are also strongly influenced by EE. Astrocyte morphology has long been known to change in response to EE, with the changes depending both on the duration of EE exposure and on the cortical layer in which the astrocytes reside (9, 24, 47). The morphological plasticity of astrocytes in response to EE appears to occur on a time-scale similar to that of neuronal changes (48). Other results support a close correlation between changes in astrocyte morphology and synapse formation (48, 49), further emphasizing the synergy between neurones and astrocytes in and around the synaptic cleft. Thus, it is not only astrocyte morphology that is changed by EE. Rather, it seems that EE affects and refines the functional relationship between astrocytes and neurones and that the morphological changes merely reflect the altered functional state of astrocytes. There is a body of evidence supporting the EE-dependent enhancement of astrocyte-synapse communication, which is of great interest in view of the fact that perisynaptic astrocytes control synaptic transmission (50–52).
Results from different animal models point to EE as a powerful modality for both preventing and recovering from CNS injuries and diseases. In the intact nervous system, EE has profound effects that can be utilized to develop and improve cognitive abilities or to resist the negative consequences of different types of stressors. To fully translate this existing knowledge into therapies, however, one must first consider some of the major determinant of the outcome of any neurorehabilitation approach, such as motivation, feeling of joy, sense of coherence, social structures, extended focused physical activities, and targeted education.
ASTROCYTES: HELPERS, DECISION-MAKERS – AND A NEW TARGET FOR REGENERATIVE STRATEGIES?
Astrocytes, intermediate filaments, and reactive gliosis
Astrocytes, the most abundant cells in the CNS, were long considered a constituent of the brain glue (glia) and remained out of the spotlight well into the 1980s. Even after their involvement in CNS pathologies (e.g. trauma, ischaemia, and neurodegenerative diseases) was suspected, they were still perceived as fulfilling at best a regulatory and homeostasis-supporting role. Astrocytes were judged to be little more than providers of nutrients and recycling units for neurotransmitters.
It was the morphological aspects of astrocytes in stroke, neurotrauma, and neurodegenerative disease that originally attracted the attention of neurologists and neuroscientists to these cells and their biology. In response to any kind of CNS injury, astrocytes change their appearance and undergo a characteristic hypertrophy of their cellular processes referred to as reactive gliosis or astrogliosis. The hallmark of this phenomenon is upregulation of the intermediate filament (IF) proteins glial fibrillary acidic protein (GFAP) and vimentin, expression of the IF proteins nestin and (in some reactive astrocytes) synemin, and alterations in the expression profiles of many other proteins (53–55).
IFs, or nanofilaments, are the least understood part of the cytoskeleton. Along with the other cytoskeletal components – microtubules and actin filaments – they provide a structural scaffold and serve as highly dynamic structures that integrate diverse intracellular functions. The family of IF proteins in vertebrates is quite large. In humans, 65 IF proteins have been identified (56, 57). IF proteins are expressed in complex patterns that are unique for each cell type and for different developmental stages. IFs were at first considered to be static structures primarily responsible for maintaining the cell shape (58, 59). However, later studies both in vitro (60, 61) and in vivo (62–65) revealed the rather dynamic nature of IFs and the existence of a dynamic equilibrium between the assembled filaments and the pool of soluble subunits (reviewed in 66).
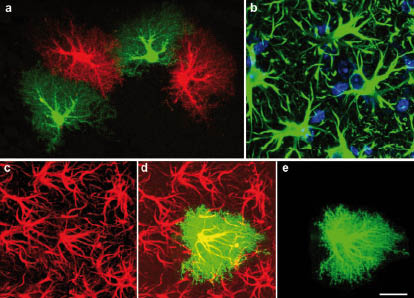
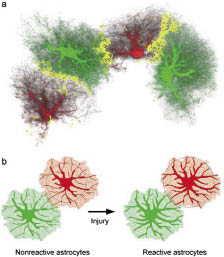
In the absence of brain or spinal cord pathology, astrocytes have many functions. However, in contrast to reactive astrocytes in the vicinity of brain lesions, their state is often described as non-reactive. In non-reactive astrocytes, IFs consist of GFAP and vimentin, while in reactive astrocytes, nestin and synemin act as additional partners in the IF network (55, 67). In reactive astrocytes lacking both GFAP and vimentin (GFAP–/–Vim–/–), no IFs are formed, and the nestin and synemin proteins that are produced stay in a non-filamentous form (68). In mature astrocytes, the major protein of the IF network is GFAP, and the vimentin level ranges from very low to intermediate, depending on the subpopulation of astrocytes (69, 70). Mature astrocytes have fine processes extending from the main cellular processes, giving each cell a characteristic bushy appearance (Fig. 1a). The IF network, however, is restricted to the main processes and the soma of astrocytes (71, 72) (Fig. 1b–e). Recently, we showed that reactive astrocytes in denervated hippocampus or near cortical lesions increase the thickness of their main cellular processes but access a volume of tissue comparable to that of non-reactive astrocytes. Despite the hypertrophy of GFAP-containing cellular processes, the interdigitation of adjacent reactive astrocytes in denervated hippocampus is minimal (73) (Fig. 2).
Fig. 1. Astrocyte morphology in mammalian brain. (a) Three-dimensional reconstruction of astrocytes. Astrocytes in the adult mouse hippocampus filled with 2 dyes. The central nervous system is divided into domains, each accessed by fine cellular processes of an astrocyte. (b) Astrocytes in the brain cortex visualized in the most common way, by antibodies against the astrocyte-specific cytoskeletal component, glial fibrillary acidic protein (GFAP). (c–e) Reactive astrocytes after dye filling and three-dimensional reconstruction. Note the typical bushy appearance of astrocytes with fine cellular processes that cannot be visualized by antibodies against GFAP (compare the central astrocyte in c, d and e). Scale bar, 20 µm. Reproduced with permission from (91).
Fig. 2. The domains of non-reactive and reactive astrocytes: a concept. (a) Interdigitation of fine cellular processes in a three-dimensional reconstruction of dye-filled astrocytes in the dentate gyrus of the hippocampus. The yellow zone shows the border area where cellular processes of 2 adjacent astrocytes interdigitate. (b) Reactive astrocytes stay within their domains, but their main cellular processes become thicker, making them visible over a greater distance (illustrated here by the grey circles). Reproduced from (73).
GFAP-positive astroglial cells may be involved in the baseline neurogenesis in the adult mammalian CNS. The findings on which this proposal was based suggested that astrocytes positively control neurogenesis in the dentate gyrus of the hippocampus and in the subventricular zone – the only 2 CNS regions in which new neurones are generated in relatively high numbers even in adults (74). Furthermore, the majority of neural stem cells in the adult CNS may at some point be GFAP positive and could therefore be defined as astroglial cells (75–78). Thus, astroglial cells might control adult neurogenesis and be the precursors of neurones added during adulthood.
Reactive gliosis, neurotrauma, and the fate of CNS transplants
To study the role of IF upregulation in reactive astrocytes in CNS injury, several trauma models were applied to mice deficient in GFAP and/or vimentin, including fine-needle injury of the brain cortex and transection of the dorsal funiculus in the upper thoracic spinal cord. The responses of wild-type, GFAP–/–, and Vim–/– mice were indistinguishable. In GFAP–/–Vim–/– mice, however, the post-traumatic glial scarring was looser and less organized, suggesting that upregulation of IFs is an important step in astrocyte activation. These findings also implied that reactive astrocytes play a role in post-traumatic healing (79). An extended healing period after CNS injury was also reported in mice in which dividing astrocytes had been ablated by GFAP-driven expression of herpes simplex virus thymidine kinase and administration of ganciclovir (80, 81). After hemisectioning of the lower thoracic spinal cord, GFAP–/–Vim–/– mice had increased axonal sprouting and better functional recovery than wild-type controls (82).
Two groups have addressed the role of astrocyte IFs in neurite outgrowth in vitro (83–85). One reported that GFAP–/–Vim–/– and GFAP–/– astrocytes are a better substrate for the outgrowth of neurites than wild-type astrocytes (83, 85). The other group found comparable neurite outgrowth when neurones were cultured on wild-type and GFAP–/– astrocytes (84). The latter finding is consistent with the normal axonal sprouting and regeneration observed after dorsal hemisectioning of the spinal cord in GFAP–/– mice (86). Recently, we reported extensive axonal regeneration in the severed optic nerve of young GFAP–/–Vim–/– mice overexpressing human Bcl2 in neurones (87), as well as reduced photoreceptor degeneration after retinal detachment in GFAP–/–Vim–/– mice (88). We suggested that the environment, in particular astrocytes but also components the immune system, are important modulators of CNS regeneration (89).
The involvement of astrocytes in synaptic regeneration after neurotrauma was studied in partially deafferented hippocampus after entorhinal cortex lesioning. Such lesions interrupt axonal connections (known as the perforant path) between the entorhinal cortex and the projection area in the outer molecular layer of the dentate gyrus of the hippocampus (90), where degenerating neurones trigger extensive reactive gliosis. The distance between these 2 regions allows assessment of astrocyte response, degeneration, and subsequent regeneration in the hippocampus, which is not directly affected by the surgery. Using this model, we showed that reactive astrocytes devoid of IFs (GFAP–/–Vim–/–) exhibited only limited hypertrophy of cell processes. Many processes of GFAP–/–Vim–/– astrocytes were shorter and less straight than those of wild-type astrocytes, although the volume of CNS tissue reached by a single astrocyte was comparable to that in wild-type mice (91). These results, along with in vitro data on the morphology of IF-depleted astrocytes in primary cultures (92), show a novel role for IFs in determining astrocyte morphology. In GFAP–/–Vim–/– mice, loss of neuronal synapses in the outer molecular layer of the hippocampal dentate gyrus was prominent 4 days after lesioning. Of particular interest was the remarkable synaptic regeneration 10 days later (14 days after injury).
Thus, the effect of reactive astrocytes after CNS trauma seems to be two-fold: reactive astrocytes play a beneficial role in the acute stage but subsequently inhibit CNS regeneration. Support for the concept of reactive gliosis as an inhibitor of post-traumatic repair and functional recovery comes also from studies of transgenic mice expressing an inhibitor of NF kappa B in astrocytes (93) or deficient in EphA4 (94).
Because of their morphology and abundance in the adult CNS, astrocytes have direct physical contact with any cell that moves from one place to another. To assess the impact of astrocyte IFs on the fate of cells migrating from neural transplants, the Chen and Pekny groups transplanted dissociated retinal cells into the retinas of adult wild-type and GFAP–/–Vim–/– mice and compared the efficiency of long-term integration of the grafts (95). In wild-type hosts, few transplanted cells migrated from the transplantation site, and few integrated into the retina. In GFAP–/–Vim–/– hosts, however, the transplanted cells effectively moved through the retina, differentiated into neurones, and integrated into the ganglion cell layer; some even extended neurites into the optic nerve. Six months after transplantation, the cells were alive and well integrated (95).
These results show that the absence of IFs in astroglial cells (astrocytes and Müller cells) of the retina increases the permissiveness of the retinal environment for integration of neural transplants. The mechanism is unknown. However, IF depletion in astroglial cells might alter their differentiation state, turning them into cells functionally similar to more immature astrocytes and therefore more supportive of CNS regeneration (96). By affecting the abundance or composition of IFs, it might be possible to control the state of cellular differentiation and thus many cellular functions. This would ultimately allow control of complex processes such as the permissiveness of the CNS for regeneration (97, 98).
INSPIRATION FOR DEVELOPING MODERN NEUROREHABILITATION APPROACHES
With the development of molecular neuroscience and technologies, such as transgenic manipulation of experimental animals and genomic and proteomic approaches, neurology develops into a modern medical discipline based on a molecular understanding of the functions of cells and tissues in health and disease. This forms a solid basis also for devising and refining novel rehabilitation strategies.
This review has presented 2 rapidly developing areas with huge potential for neurorehabilitation. One, the EE, has been utilized in rehabilitation for centuries. Only now, however, can its full potential be comprehensively demonstrated and quantitatively assessed in diverse experimental systems. This potential has not been fully realized, or implemented in ways that can be expected to provide maximal therapeutic effects and benefits. The application of EE is by no means limited to rehabilitation after stroke or even to neurorehabilitation as such. When applied in the form of tailored programs with full emphasis on motivation, it might become a major component of prevention programs.
The second area discussed here – astrocytes as a novel target for regeneration promoting treatment paradigms – is equally exciting. This concept represents a shift in the current, admittedly largely neurocentric, thinking in neuroscience and neurorehabilitation. Astrocytes as a cellular target for brain and spinal cord repair are now at the centre of molecular neuroscience and modern neuropharmacology. Their increasing recognition within neurology is opening up new opportunities to explore the synergy between traditional and novel neurorehabilitation in the future.
ACKNOWLEDGEMENTS
The authors thank Drs Michelle Anderson, Marcela Pekna and Ulrika Wilhelmsson for their input into this review. This work was supported by grants from the Swedish Medical Research Council (project 20114 and 11548), The Region of Västra Götaland (RUN), Swedish Stroke Foundation, Torsten and Ragnar Söderberg Foundations, Heart-Lung Foundation, the Swedish Society for Medicine, W. and M. Lundgren Foundation, John and Brit Wennerström’s Foundation for Neurological Research, Foundation Edit Jacobson’s Donation Fund, The Rune and Ulla Amlövs Foundation, The Axel Linders Foundation Trygg-Hansa, Hjärnfonden, ALF Göteborg and Åhlén-stiftelsen.
REFERENCES
1. Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med 2005; 352: 1677–1684.
2. Gresham GE, Fitzpatrick TE, Wolf PA, McNamara PM, Kannel WB, Dawber TR. Residual disability in survivors of stroke – the Framingham study. N Engl J Med 1975; 293: 954–956.
3. Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature 1980; 284: 264–265.
4. David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 1981; 214: 931–933.
5. Gimenez y Ribotta M, Menet V, Privat A. The role of astrocytes in axonal regeneration in the mammalian CNS. Prog Brain Res 2001; 132: 587–610.
6. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002; 8: 963–970.
7. Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 2002; 52: 802–813.
8. Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci 2003; 24: 171–189.
9. Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol 2004; 1: 351–363.
10. Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Ann Rev Neurosci 2005; 28: 377–401.
11. Hummel FC, Cohen LG. Drivers of brain plasticity. Curr Opin Neurol 2005; 18: 667–674.
12. Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol 2004; 61: 1844–1848.
13. Hebb D. The effects of early experience on problem-solving at maturity. Am Psychol 1947; 2: 306–307.
14. Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002). Prog Neurobiol 2004; 72: 167–182.
15. Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res 1978; 153: 563–576.
16. Mohammed AK, Jonsson G, Archer T. Selective lesioning of forebrain noradrenaline neurons at birth abolishes the improved maze learning performance induced by rearing in complex environment. Brain Res 1986; 398: 6–10.
17. Whishaw IQ, Zaborowski JA, Kolb B. Postsurgical enrichment aids adult hemidecorticate rats on a spatial navigation task. Behav Neural Biol 1984; 42: 183–190.
18. Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol 2000; 164: 45–52.
19. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002; 25: 295–301.
20. Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 2006; 7: 697–709.
21. Walsh RN. Effects of environmental complexity and deprivation on brain anatomy and histology: a review. Int J Neurosci 1981; 12: 33–51.
22. Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci 2001; 21: 5272–5280.
23. Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab 2002; 22: 89–96.
24. Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res 1987; 424: 320–332.
25. Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol 1999; 39: 569–578.
26. Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997; 386: 493–495.
27. Gobbo OL, O’Mara SM. Impact of enriched-environment housing on brain-derived neurotrophic factor and on cognitive performance after a transient global ischemia. Behav Brain Res 2004; 152: 231–241.
28. Dahlqvist P, Zhao L, Johansson IM, Mattsson B, Johansson BB, Seckl JR, et al. Environmental enrichment alters nerve growth factor-induced gene A and glucocorticoid receptor messenger RNA expression after middle cerebral artery occlusion in rats. Neurosci 1999; 93: 527–535.
29. van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington’s in mice. Nature 2000; 404: 721–722.
30. Wexler NS, Lorimer J, Porter J, Gomez F, Moskowitz C, Shackell E, et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc Natl Acad Sci USA 2004; 101: 3498–3503.
31. Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci 2005; 25: 4217–4221.
32. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 1994; 271: 1004–1010.
33. Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Chen CH, et al. Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group members. Proc Natl Acad Sci USA 2001; 98: 3440–3445.
34. Mayeux R. Epidemiology of neurodegeneration. Ann Rev Neurosci 2003; 26: 81–104.
35. Bezard E, Dovero S, Belin D, Duconger S, Jackson-Lewis V, Przedborski S, et al. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci 2003; 23: 10999–11007.
36. Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med 1999; 5: 448–453.
37. Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci 2004; 24: 1245–1254.
38. Dahlqvist P, Rönnback A, Bergström SA, Söderström I, Olsson T. Environmental enrichment reverses learning impairment in the Morris water maze after focal cerebral ischemia in rats. Eur J Neurosci 2004; 19: 2288–2298.
39. Hicks RR, Zhang L, Atkinson A, Stevenon M, Veneracion M, Seroogy KB. Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neurosci 2002; 112: 631–637.
40. Kozlowski DA, Nahed BV, Hovda DA, Lee SM. Paradoxical effects of cortical impact injury on environmentally enriched rats. J Neurotrauma 2004; 21: 513–519.
41. Passineau MJ, Green EJ, Dietrich WD. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp Neurol 2001; 168: 373–384.
42. Maegele M, Lippert-Gruener M, Ester-Bode T, Garbe J, Bouillon B, Neugebauer E, et al. Multimodal early onset stimulation combined with enriched environment is associated with reduced CNS lesion volume and enhanced reversal of neuromotor dysfunction after traumatic brain injury in rats. Eur J Neurosci 2005; 21: 2406–2418.
43. Beaulieu CL. Rehabilitation and outcome following pediatric traumatic brain injury. The Surg Clin North Am 2002; 82: 393–408.
44. Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB. Enriched environment after focal cortical ischemia enhances the generation of astroglia and NG2 positive polydendrocytes in adult rat neocortex. Exp Neurol 2006; 199: 113–121.
45. Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB. Effects of cortical ischemia and postischemic environmental enrichment on hippocampal cell genesis and differentiation in the adult rat. J Cereb Blood Flow Metab 2002; 22: 852–860.
46. Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, et al. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci USA 2000; 97: 12880–12884.
47. Sirevaag AM, Greenough WT. Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Res 1991; 540: 273–278.
48. Jones TA, Greenough WT. Ultrastructural evidence for increased contact between astrocytes and synapses in rats reared in a complex environment. Neurobiol Learn Mem 1996; 65: 48–56.
49. Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science 2001; 291: 657–661.
50. Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 2007; 13: 54–63.
51. Zhang Q, Haydon PG. Roles for gliotransmission in the nervous system. J Neural Transm 2005; 112: 121–125.
52. Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci 2001; 2: 185–193.
53. Hernandez MR, Agapova OA, Yang P, Salvador-Silva M, Ricard CS, Aoi S. Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia 2002; 38: 45–64.
54. Eddleston M, Mucke L. Molecular profile of reactive astrocytes–implications for their role in neurologic disease. Neurosci 1993; 54: 15–36.
55. Jing R, Wilhelmsson U, Goodwill W, Li L, Pan Y, Pekny M, Skali O. Synemin is expressed in reactive astrocytes in neurotrauma and interacts differentially with viemtin and GFAP intermediate filament networks. J Cell Sci 2007; 120: 1267–1277.
56. Herrmann H, Hesse M, Reichenzeller M, Aebi U, Magin TM. Functional complexity of intermediate filament cytoskeletons: from structure to assembly to gene ablation. Int Rev Cytol 2003; 223: 83–175.
57. Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu Rev Biochem 2004; 73: 749–789.
58. Rueger DC, Huston JS, Dahl D, Bignami A. Formation of 100 A filaments from purified glial fibrillary acidic protein in vitro. J Mol Biol 1979; 135: 53–68.
59. Renner W, Franke WW, Schmid E, Geisler N, Weber K, Mandelkow E. Reconstitution of intermediate-sized filaments from denatured monomeric vimentin. J Mol Biol 1981; 149: 285–306.
60. Angelides KJ, Smith KE, Takeda M. Assembly and exchange of intermediate filament proteins of neurons: neurofilaments are dynamic structures. J Cell Biol 1989; 108: 1495–1506.
61. Nakamura Y, Takeda M, Angelides KJ, Tada K, Hariguchi S, Nishimura T. Assembly, disassembly, and exchange of glial fibrillary acidic protein. Glia 1991; 4: 101–110.
62. Miller RK, Vikstrom K, Goldman RD. Keratin incorporation into intermediate filament networks is a rapid process. J Cell Biol 1991; 113: 843–855.
63. Wiegers W, Honer B, Traub P. Microinjection of intermediate filament proteins into living cells with and without preexisting intermediate filament network. Cell Biol Int Rep 1991; 15: 287–296.
64. Vikstrom KL, Lim SS, Goldman RD, Borisy GG. Steady state dynamics of intermediate filament networks. J Cell Biol 1992; 118: 121–129.
65. Yoon M, Moir RD, Prahlad V, Goldman RD. Motile properties of vimentin intermediate filament networks in living cells. J Cell Biol 1998; 143: 147–157.
66. Goldman RD, Chou YH, Prahlad V, Yoon M. Intermediate filaments: dynamic processes regulating their assembly, motility, and interactions with other cytoskeletal systems. FASEB J 1999; 13 Suppl 2: S261–265.
67. Pekny M, Eliasson C, Chien CL, Kindblom LG, Liem R, Hamberger A, et al. GFAP-deficient astrocytes are capable of stellation in vitro when cocultured with neurons and exhibit a reduced amount of intermediate filaments and an increased cell saturation density. Exp Cell Res 1998; 239: 332–343.
68. Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, et al. Intermediate filament protein partnership in astrocytes. J Biol Chem 1999; 274: 23996–24006.
69. Pixley SK, Kobayashi Y, de Vellis J. A monoclonal antibody against vimentin: characterization. Brain Res 1984; 317: 185–199.
70. Shaw G, Osborn M, Weber K. An immunofluorescence microscopical study of the neurofilament triplet proteins, vimentin and glial fibrillary acidic protein within the adult rat brain. Eur J Cell Biol 1981; 26: 68–82.
71. Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 2002; 22: 183–192.
72. Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci 2004; 22: 73–86.
73. Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci USA 2006; 103: 17513–17518.
74. Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature 2002; 417: 39–44.
75. Morshead CM, Garcia AD, Sofroniew MV, van Der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci 2003; 18: 76–84.
76. Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97: 703–716.
77. Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci 2003; 23: 2824–2832.
78. Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA 2000; 97: 13883–13888.
79. Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, et al. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol 1999; 145: 503–514.
80. Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 1999; 2: 297–308.
81. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 2004; 24: 2143–2155.
82. Menet V, Prieto M, Privat A, Gimenez y Ribotta M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci USA 2003; 100: 8999–9004.
83. Menet V, Gimenez y Ribotta M, Chauvet N, Drian MJ, Lannoy J, Colucci-Guyon E, et al. Inactivation of the glial fibrillary acidic protein gene, but not that of vimentin, improves neuronal survival and neurite growth by modifying adhesion molecule expression. J Neurosci 2001; 21: 6147–6158.
84. Xu K, Malouf AT, Messing A, Silver J. Glial fibrillary acidic protein is necessary for mature astrocytes to react to beta-amyloid. Glia 1999; 25: 390–403.
85. Menet V, Gimenez YRM, Sandillon F, Privat A. GFAP null astrocytes are a favorable substrate for neuronal survival and neurite growth. Glia 2000; 31: 267–272.
86. Wang X, Messing A, David S. Axonal and nonneuronal cell responses to spinal cord injury in mice lacking glial fibrillary acidic protein. Exp Neurol 1997; 148: 568–576.
87. Cho KS, Yang L, Lu B, Feng Ma H, Huang X, Pekny M, et al. Re-establishing the regenerative potential of central nervous system axons in postnatal mice. J Cell Sci 2005; 118: 863–872.
88. Nakazawa T, Takeda M, Lewis GP, Cho K-S, Jianwei J, Wilhelmsson U, et al. Attenuated glial reactions and photoreceptor degeneration after retinal detachment in mice deficient in glial fibrillary acidic protein and vimentin Invest Ophthal Vis Sci 2007 (in press).
89. Rahpeymai Y, Hietala MA, Wilhelmsson U, Fotheringham A, Davies I, Nilsson AK, et al. Complement: a novel factor in basal and ischemia-induced neurogenesis. EMBO J 2006; 25: 1364–1374.
90. Turner DA, Buhl EH, Hailer NP, Nitsch R. Morphological features of the entorhinal-hippocampal connection. Prog Neurobiol 1998; 55: 537–562.
91. Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci 2004; 24: 5016–5021.
92. Lepekhin EA, Eliasson C, Berthold CH, Berezin V, Bock E, Pekny M. Intermediate filaments regulate astrocyte motility. J Neurochem 2001; 79: 617–625.
93. Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 2005; 202: 145–156.
94. Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci 2004; 24: 10064–10073.
95. Kinouchi R, Takeda M, Yang L, Wilhelmsson U, Lundkvist A, Pekny M, et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci 2003; 6: 863–868.
96. Emsley JG, Arlotta P, Macklis JD. Star-cross’d neurons: astroglial effects on neural repair in the adult mammalian CNS. Trends Neurosci 2004; 27: 238–240.
97. Pekny M, Pekna M, Wilhelmsson U, Chen DF. Response to Quinlan and Nilsson: astroglia sitting at the controls? Trends Neurosci 2004; 27: 243–244.
98. Quinlan R, Nilsson M. Reloading the retina by modifying the glial matrix. Trends Neurosci 2004; 27: 241–242.