Thilo O. Kromer, MMuscPhty1,3, Rob A. de Bie, PhD2,3 and Caroline H. G. Bastiaenen, PhD2,3
From the 1School of Therapeutic Sciences, Department of Physiotherapy, SRH University Heidelberg, Heidelberg, Germany and 2Department of Epidemiology, 3Caphri Research School, Maastricht University, Maastricht, The Netherlands
OBJECTIVE: To investigate the effect of individualized manual physiotherapy and exercises compared with individualized exercises alone in patients with shoulder impingement syndrome.
DESIGN: Randomized controlled trial.
SUBJECTS: Patients with shoulder impingement of more than 4 weeks’ duration.
METHODS: Patients in the intervention group were treated with individually adapted exercises and examination-based physiotherapy. Controls were treated with individually adapted exercises only. Both groups had 10 treatment sessions over a period of 5 weeks and subsequently continued their exercises at home for another 7 weeks. Results were analysed at 5 and 12 weeks after the start of the study. Primary outcome measures were: Shoulder Pain and Disability Index, and Patient’s Global Impression of Change. Secondary outcome measures were: mean weekly pain score; Generic Patient-Specific Scale; and Patients’ Satisfaction with Treatment.
RESULTS: A total of 46 patients were randomized to the intervention group and 44 to the control group. Although both groups showed significant improvements, there was no difference between groups for the primary and secondary outcomes at any time. Only the results for mean pain differed at 5 weeks in favour of the intervention group.
CONCLUSION: Individually adapted exercises were effective in the treatment of patients with shoulder impingement syndrome. Individualized manual physiotherapy contributed only a minor amount to the improvement in pain intensity. However, further research is necessary to confirm these results before definite recommendations can be made.
Key words: shoulder impingement syndrome; physiotherapy; exercise therapy; intervention; randomized controlled trial.
J Rehabil Med 2013; 45: 488–497
Guarantor’s address: Caroline H. G. Bastiaenen, Department of Epidemiology, Maastricht University, PO BOX 616, NL-6200 MD Maastricht, The Netherlands. E-mail: chg.bastiaenen@maastrichtuniversity.nl
Accepted Dec 19, 2012; Epub ahead of print Apr 12, 2013
Introduction
Shoulder pain is a common complaint seen by health professionals (1, 2), with an incidence of 9.5 per 1,000 patients presenting to primary care and a point prevalence of 7–26% (3, 4). Shoulder pain has a considerable effect on health (4, 5), and seems to have a recurrent nature, with low recovery rates even 3 years after onset (1, 6, 7). Although no standardized classification for shoulder complaints exists, most shoulder patients show clinical signs of subacromial impingement (2, 4). Subacromial impingement syndrome (SIS) occurs due to a mechanical disturbance within the subacromial space. It is characterized by pain and functional restrictions, mostly during overhead activities (8).
Physiotherapy is an often prescribed measure for the treatment of shoulder disorders (2, 9). Particularly for SIS the use of exercise therapy to improve muscle strength, flexibility and coordination of the rotator cuff and the shoulder girdle muscles have been reported in several studies (10–15). Combining exercises with manual therapy to specifically influence structural components of the shoulder complex and spine seems to be even more effective and is therefore recommended in secondary literature (16, 17). However, the available evidence for the effect of these interventions is limited due to small sample sizes and other methodological flaws. Recent systematic reviews on this topic emphasize the need for more high-quality trials, especially of combination of modalities to reflect common practice (16, 18–20).
This randomized controlled trial investigated the effect in patients with SIS of individualized manual physiotherapy combined with an individualized exercise programme on pain and functioning, compared with individualized exercises alone. The study design has been published previously (21). To our knowledge this is the first trial of this type in the German population.
Methods
Participants
Participants were recruited by referral from general practitioners or orthopaedic surgeons to physiotherapy because of shoulder complaints. They were screened for the clinical presentation of SIS by trained physiotherapists, with the following eligibility criteria:
Inclusion criteria were: (i) age between 18 and 75 years, (ii) symptoms for at least 4 weeks, (iii) main complaints in the glenohumeral joint region or the proximal arm, (iv) presence of one of the following signs indicating SIS: Neer impingement sign, Hawkins-Kennedy impingement test, painful arc with active abduction or flexion, and (v) pain during one of the following resistance tests: external rotation, internal rotation, abduction, or flexion.
Exclusion criteria were: (i) mean 24-h pain of 8/10 or more on a visual numeric rating scale (VNRS), (ii) primary scapulothoracic dysfunction due to paresis, (iii) diagnosed instability or previous history of dislocation, (iv) adhesive capsulitis (frozen shoulder), (v) more than one-third restriction of elevation compared with the unaffected side, (vi) substantial shoulder weakness or loss of active shoulder function, (vii) shoulder surgery in the last 12 months on the involved side, (viii) reproduction of symptoms with active or passive cervical movements, (ix) neurological involvement with sensory and muscular deficit, (x) inflammatory joint disease (e.g. rheumatoid arthritis), (xi) diabetes mellitus, (xii) intake of psychotherapeutic drugs, (xiii) compensation claims, and (xiv) inability to understand written or spoken German.
Ethical approval was granted by the ethics committee of the Ludwig-Maximilians-University Munich, Germany (project number 018-10), and all patients gave informed consent.
Trial registration. Current Controlled Trials ISRCTN86900354.
Inclusion process and randomization
After signing informed consent and baseline assessment, eligible participants were randomly allocated to treatment groups in blocks of 6 using central blinded randomization. To guarantee allocation concealment, therapists received the information about patient allocation immediately before the first treatment by the Department of Epidemiology, Maastricht University.
Interventions
The intervention group received individually adapted exercises (IAEX) plus individualized manual physiotherapy (IMPT), the control group received IAEX only. A detailed description of the interventions is provided in the published protocol for this study (21) and in Appendix I. Treatment was provided in 6 outpatient physiotherapy clinics by 12 experienced and trained physiotherapists with an international qualification for manual therapy according to International Federation Orthopaedic Manipulative Physical Therapists standard and a mean post-qualification experience of more than 23 years (range 18–28 years). Participants received 10 treatment sessions within 5 weeks. To guarantee an equal instruction of the exercise programme in both groups, and to be able to identify the additional effect of the IMPT, the time-frame for treatment was 15–20 min for the control group and 20–30 min for the intervention group. Afterwards both groups continued their exercise programme 3 times a week for a further 7 weeks.
Therapists’ compliance with the protocol. Compliance of therapists with the treatment guidelines was monitored with the help of the examination and treatment records, group meetings and regular interviews.
Outcome measures
Patients were assessed at baseline, and after the intervention period at 5 weeks and at 12 weeks after the start of the study. Primary outcome measures were the Shoulder Pain and Disability Index (SPADI) (22) and Patient’s Global Impression of Change (PGIC). An improvement of 11 points in the total SPADI score (23) and a statement of “slightly better” in the PGIC were considered as minimum clinically important changes. As secondary outcome measures we used the Generic Patient-Specific Scale (GPSS) (24), the mean weekly pain score and Patients’ Satisfaction with Treatment (PST). For the GPSS a mean score across all activities was calculated. A minimum change of 30% was considered as a clinically important improvement (25, 26). For mean pain an improvement of 2 points or more on a visual numeric rating scale (VNRS) was defined as a clinically important difference (25, 26). Satisfaction with treatment was also rated on an 11-point VNRS, with 10 defined as “completely satisfied” and 0 as “completely dissatisfied”. A more detailed description of all outcome measures is given in the study protocol. To be able to analyse the possible influence of other important factors on our main outcome all patients completed a modified version of the Fear Avoidance Beliefs Questionnaire (FABQ), the Pain Catastrophizing Scale (PCS), and answered a question about their expectations of treatment outcome at baseline, also scored on a VNRS, with higher scores reflecting more positive expectations.
Compliance with treatment was assessed with the shoulder log book. Treatment compliance included the number of attended treatment visits out of a maximum of 10 and the frequency of the home exercises. Demographic data including age, sex, height, weight, profession, sports activities, information about medication intake, sick leave, severity and duration of symptoms and previous episodes of shoulder pain were also documented.
Sample size calculation
Power calculation resulted in an estimated sample size of 90 participants (45 per group) to detect a 13-point difference in SPADI score. The assumed standard deviation (SD) was set to 20 points, based on the results of other studies (27–30). Alpha was set to 0.05; statistical power to 80% given an expected drop-out rate of 15%.
Data analysis
Descriptive statistics for demographic and clinical characteristics were used, for baseline results of outcome measures and other potentially confounding variables for both groups and the total group. Data for work classification, working hours per week, and sick leave were analysed only for patients who were in work. Work was classified according to the estimated physical load for the upper extremity of the patient’s profession. Examples for class 1 professions are accountants, secretaries, or school teachers; for class 2 housewives, nurses, or retail dealers; and for class 3 manual workers, such as carpenters, gardeners, or mechanics. Differences after 5 and 12 weeks were calculated for between-group comparisons and within-group results according to the “intention-to-treat principle”. For between-group analysis mean differences between groups, their SDs and 95% confidence intervals (95% CI) were calculated for each clinical outcome measure. Within-group results were calculated by subtracting the 5-week results from baseline values and the 12-week from the 5-week values. Influence of baseline differences and other potentially influencing factors based on literature on the main outcome measure were assessed in a multivariable linear regression analysis. Statistical significance was set to p ≤ 0.05. Due to the nature of the intervention it was impossible to blind therapist and participants. However, we blinded therapists for the control group to all clinical information about their patients. Measurements of outcome were also blinded because therapists were not involved in this process, and patients were kept naive as to their allocation. Statistical analyses were performed using IBM SPSS Statistics 19.
Results
Recruitment process
A total of 188 patients was assessed for eligibility over an 18-month period. Of these, 55 patients did not fulfil the eligibility criteria, 33 refused to participate, and 10 were not included due to other reasons (3 moved, 4 were no prescribed physiotherapy, 3 could not participate in continuous treatment due to frequent business travel abroad). Finally, 90 participants were randomly allocated, with 44 patients in the control group (IAEX) and 46 patients in the intervention group (IAEX + IMPT). At 5 weeks all patients were analysed with no loss to follow-up. At 12 weeks 2 patients in the intervention group discontinued treatment, 1 without giving a reason, the other reported that treatment took too much effort. The recruitment process is summarized in Fig. 1.
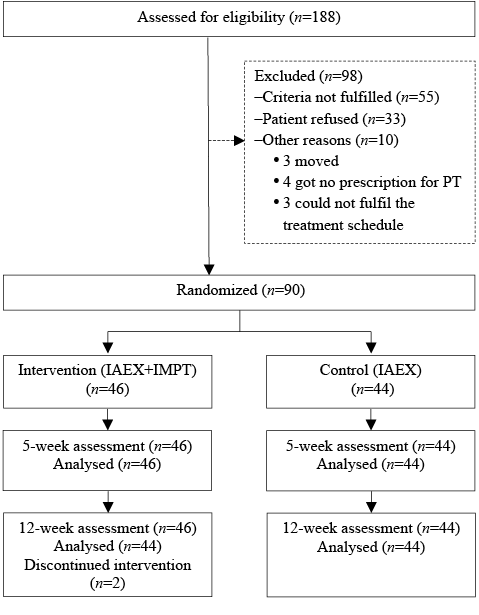
Fig. 1. Inclusion process. PT: physical therapist; IAEX: individually adapted exercises; IMPT: individualized manual physiotherapy.
No significant differences were found in demographic and clinical baseline characteristics between groups, except for sports hours per week, overall duration of symptoms, total FABQ, and the FABQ activity subscale. Baseline characteristics for the total group, the intervention and control group are displayed in Tables I and II.
|
Table I. Baseline demographic data and baseline results of the questionnaires |
|||
|
Intervention group (n = 46) |
Control group (n = 44) |
Total group (n = 90) |
|
|
Age, years, mean (SD) |
50.1 (12.2) |
53.7 (9.9) |
51.8 (11.2) |
|
18–29 years, n (%) |
2 (4.3) |
0 (0.0) |
2 (2.2) |
|
30–44 years, n (%) |
13 (28.3) |
8 (18.2) |
21 (23.4) |
|
45–59 years, n (%) |
21 (45.7) |
20 (45.4) |
41 (45.5) |
|
> 60 years, n (%) |
10 (21.7) |
16 (36.4) |
26 (28.9) |
|
Gender (female), n (%) |
22 (47.8) |
24 (54.5) |
46 (51.1) |
|
BMI, mean (SD) |
25.3 (3.7) |
26.8 (4.3) |
26.0 (4.1) |
|
Classification of physical work loada, n (%) |
(n = 40) |
(n = 38) |
(n = 78) |
|
Low |
16 (40.0) |
19 (50.0) |
35 (44.9) |
|
Medium |
18 (45.0) |
13 (34.2) |
31 (39.7) |
|
High |
6 (15.0) |
6 (15.8) |
12 (15.4) |
|
Working hours per weeka, mean (SD) |
32.2 (13.8) |
37.2 (10.7) |
34.6 (12.6) |
|
Days of sick leavea, mean (SD) |
0.1 (0.6) |
1.1 (4.1) |
0.6 (2.9) |
|
Sports hours per week, n (%) |
|||
|
0–2 h |
13 (28.3) |
21 (47.4) |
34 (37.8) |
|
3–5 h |
33 (71.7) |
23 (52.6) |
56 (62.2) |
|
Duration of the current episode in weeks, mean (SD) |
27.4 (28.4) |
40.8 (53.4) |
33.9 (42.8) |
|
Overall duration of shoulder pain in weeks, mean (SD) |
136.9 (198.5) |
71.3 (68.7) |
104.8 (152.6) |
|
Number of episodes during the last 12 months, n (%) |
|||
|
1–3 (including the current one) |
37 (80.4) |
38 (86.4) |
75 (83.3) |
|
> 3 |
9 (19.6) |
6 (13.6) |
15 (16.7) |
|
Pain score, mean (SD) |
5.2 (1.8) |
5.0 (1.8) |
5.1 (1.8) |
|
SPADI total score, mean (SD) |
39.7 (17.2) |
41.3 (17.0) |
40.4 (17.0) |
|
SPADI sub-score for pain, mean (SD) |
47.8 (18.8) |
49.6 (17.3) |
48.7 (18.0) |
|
SPADI sub-score for function, mean (SD) |
31.5 (18.6) |
32.9 (19.3) |
32.2 (18.9) |
|
GPSS score, mean (SD) |
4.1 (1.8) |
4.0 (1.7) |
4.0 (1.7) |
|
FABQ total score, mean (SD) |
36.4 (17.4) |
28.7 (16.7) |
32.7 (17.4) |
|
FABQ sub-score for physical activity, mean (SD) |
15.9 (4.1) |
13.3 (5.3) |
14.6 (4.9) |
|
FABQ sub-score for work, mean (SD) |
13.4 (10.3) |
10.8 (9.5) |
12.1 (9.9) |
|
PCS total score, mean (SD) |
12.4 (9.7) |
10.4 (7.1) |
11.4 (8.5) |
|
PCS sub-score for rumination, mean (SD) |
4.6 (3.9) |
3.8 (3.0) |
4.2 (3.5) |
|
PCS sub-score for magnification, mean (SD) |
3.1 (2.6) |
2.5 (1.9) |
2.8 (2.3) |
|
PCS sub-score for helplessness, mean (SD) |
4.7 (4.2) |
4.1 (3.3) |
4.4 (3.8) |
|
PET, mean (SD) |
8.4 (1.6) |
8.7 (1.3) |
8.5 (1.5) |
|
aOnly participants who are in work; 40 and 38, respectively. SD: standard deviation; BMI: body mass index; SPADI: Shoulder Pain and Disability Index; GPSS: Generic Patient Specific Scale; FABQ: Fear Avoidance Beliefs Questionnaire; PCS: Pain Catastrophizing Scale; PET: Patients Expectancies of Treatment Outcome. |
|||
|
Table II. Baseline clinical test results |
|||||
|
Clinical tests (positive results) |
Intervention group (n = 46) n (%) |
Control group (n = 44) n (%) |
Total group (n = 90) n (%) |
||
|
Painful arc |
44 (95.7) |
43 (97.7) |
87 (96.7) |
||
|
Hawkins-Kennedy test |
34 (73.9) |
33 (75.0) |
67 (74.4) |
||
|
Neer compression test |
38 (82.6) |
42 (95.5) |
80 (88.9) |
||
|
ER lag sign |
0 (0.0) |
1 (2.3) |
1 (1.1) |
||
|
Lift off test |
0 (0.0) |
1 (2.3) |
1 (1.1) |
||
|
Hornblower’s sign |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||
|
Restriction of caudal glide |
39 (84.8) |
38 (86.4) |
77 (85.6) |
||
|
Restriction of posterior glide |
35 (76.1) |
38 (86.4) |
73 (81.1) |
||
|
Restriction of passive elevation (up to 20º) |
14 (30.4) |
14 (31.8) |
28 (31.1) |
||
|
Restriction of passive ER (up to 15º) |
11 (23.9) |
13 (29.5) |
24 (26.7) |
||
|
Comparable signs of the cervical spine |
27 (58.7) |
23 (52.3) |
50 (55.6) |
||
|
ER: external rotation. |
|||||
Study power
Our power calculation was based on a 13-point difference in SPADI score and an estimated SD of 20 points. With a mean improvement of 14.9 (SD 18.5) points on the SPADI for the total group and no drop-outs, this study has sufficient power.
Shoulder log books
From a total of 90 participants 89 (98.9%) returned a complete log book after 5 weeks and 85 (94.4%) after 12 weeks, of which 3 were incomplete and could only be partially analysed.
Sick leave
Of the patients who were at work (n = 78) 7.7% (n = 6) were responsible for all days of sick leave during the 5-week treatment period. Only 1 patient from the intervention group had 12 sick days, compared with 5 patients from the control group with a total of 58 days. During the home exercise period data were available for 73 participants. Again, 1 patient in the intervention group was responsible for 4 sick days, compared with 3 patients with 41 sick days in the control group.
Exercise frequency
Mean exercise frequency per week including the two supervised sessions for both groups was 5.5 (SD 1.3) and during the home exercise period 3.8 (SD 1.6) for the intervention and 3.9 (SD 1.8) for the control group.
Additional diagnostics, medication and co-interventions
Baseline to week 5. Five patients in the intervention and 7 in the control group additionally received non-steroidal anti-inflammatory drugs (NSAIDs) from their general practitioner. 5 in the intervention and nobody in the control group had an injection with cortisone. One patient in the intervention and 2 in the control group had self-paid massages for their back during the 5 weeks, 1 patient in each group made use of a soothing ointment containing dimethyl sulphoxide and heparin (Dolobene®). One patient in the intervention group had 5 treatments with electrotherapy. For further diagnosis, 1 patient in the control group and 3 patients in the intervention group had magnetic resonance imaging (MRI), with 1 of them also having 2 X-rays.
Weeks 6–12. In the intervention group 2 had a cortisone injection, 4 received NSAIDs and 2 participants had a combination of both. Eleven patients received a total of 39 additional physiotherapy treatments and 1 had a diagnostic MRI. In the control group, 3 had a cortisone injection, 4 NSAIDs and 1 participant a combination of both; only 2 patients had a total of 12 additional treatments.
Therapists’ compliance with the protocol
All patients in the intervention group were examined and treated according to the initial instructions. They received passive manual mobilization techniques for the shoulder complex, the cervical or thoracic spine, and self-mobilization exercises to intensify the effect of the passive techniques. Patients were informed about the influence of their daily activities on symptoms and healing, instructed on how to avoid, modify or compensate their most provocative activities at work and during leisure time, and focused on an upright posture. Patients in the intervention group also performed specific exercises to improve scapular setting, control and scapulohumeral rhythm. Therapists for the control group remained blinded to the clinical examination results. However, due to illness of 1 of the therapists, 2 patients in the control group were supervised by the therapist for the intervention group for half of their contact sessions. During the first 3 treatment sessions it was difficult for therapists for the intervention group to instruct the exercises in addition to the individualized manual treatments within the given mean time-frame of approximately 25 min. They estimated their time for the exercise instructions with approximately 35–40% (9–10 min). Therefore they were allowed to extend the exercise instruction time if necessary in order to guarantee sufficient instruction of the exercises in comparison with the control group for the first 3 sessions. Therapists for the control group felt uneasy about starting treatment without having any clinical baseline information, even though clear instructions were given.
Efficacy analysis
Total SPADI score and sub-scores. Both groups improved significantly after 5 and 12 weeks in total SPADI score and its sub-scores (Table III). No difference between groups in any of the SPADI scores could be detected (Table IV). Due to baseline differences between groups overall duration of symptoms, total FABQ, the FABQ activity sub-score and other potentially influencing baseline covariates identified from the literature were entered into a univariate linear regression analysis to check their influence on group differences in SPADI. Significant relevant covariates (p < 0.05) were then combined in a multivariable regression analysis, which had a certain influence but did not change group difference to a significant level.
Mean pain score and the generic patient-specific scale. Both groups improved significantly in pain levels (p = 0.000) and GPSS scores (p = 0.000) within the first 5 weeks, but only the control group showed further improvement up to week 12 (Table III). However, the difference between groups was not significant (Table IV). An overview about the activities chosen by the participants for the GPSS is given in Appendix II. Activities involving upward-directed movements are clearly the most disabled, followed by lying on the affected side, sports activities and dressing. Between-group results and additional within-group comparisons are shown in Tables III and IV.
|
Table III. Results after 5 and 12 weeks for within-group comparison |
||||||||||
|
Outcomes |
Week 0 |
Week 5 |
Week 12 |
Difference within groups at 5 weeks |
Difference within groups between 5 and 12 weeks |
|||||
|
IG (n = 46) Mean (SD) |
CG (n = 44) Mean (SD) |
IG (n = 46) Mean (SD) |
CG (n = 44) Mean (SD) |
IG (n = 44) Mean (SD) |
CG (n = 44) Mean (SD) |
IG (n = 46) Mean (SD) [95% CI] |
CG (n = 44) Mean (SD) [95% CI] |
IG (n = 44) Mean (SD) [95% CI] |
CG (n = 44) Mean (SD) [95% CI] |
|
|
SPADI (0–100) |
39.7 (17.2) |
41.3 (17.0) |
23.5 (17.5) |
26.8 (17.8) |
16.1 (17.2) |
19.8 (19.5) |
16.2 (18.2)*** [10.8 – 21.6] |
14.4 (17.1)*** [9.2–19.6] |
7.5 (12.3)*** [3.7–11.2] |
7.0 (13.8)** [2.8–11.2] |
|
Pain SPADI (0–100) |
47.8 (18.8) |
49.6 (17.3) |
29.8 (21.1) |
31.5 (18.8) |
20.1 (19.7) |
24.1 (21.7) |
18.0 (20.2)*** [12.0 – 24.0] |
18.0 (21.4)*** [11.5–24.5] |
9.8 (15.2)*** [5.2–14.4] |
7.4 (16.6)** [2.4–12.5] |
|
Function SPADI (0–100) |
31.5 (18.6) |
32.9 (19.3) |
17.1 (15.0) |
22.1 (18.1) |
12.1 (15.4) |
15.5 (18.1) |
14.4 (18.8)*** [8.8–20.0] |
10.8 (15.8)*** [6.0–15.6] |
5.1 (10.8)** [1.9–8.4] |
6.7 (12.6)*** [2.8–10.5] |
|
Pain (0–10) |
5.2 (1.8) |
5.0 (1.8) |
2.9 (1.6) |
3.3 (1.6) |
2.3 (1.8) |
2.3 (1.8) |
2.3 (1.8)*** [1.7–2.8] |
1.6 (2.3)*** [1.0–2.3] |
0.6 (1.5)* [0.1–1.0] |
1.0 (1.7)*** [0.5–1.5] |
|
GPSS (0–10) |
4.1 (1.8) |
4.0 (1.7) |
7.1 (2.0) |
6.3 (2.0) |
7.3 (2.5) |
7.4 (2.0) |
3.0 (2.3)*** [2.3–3.7] |
2.3 (2.2)*** [1.6–3.0] |
0.3 (1.8) [–0.27–0.81] |
1.1 (2.0)*** [0.5–1.7] |
|
*p = 0.05; **p = 0.01; ****p = 0.001. IG: intervention group; CG: control group; SPADI: Shoulder Pain and Disability Index; GPSS: Generic Patient-Specific Scale; SD: standard deviation, CI: confidence interval. |
||||||||||




|
Table IV. Results after 5 and 12 weeks for between-group comparisons |
||||
|
Outcomes |
Difference between groups at 5 weeks (change scores 0–5 weeks) |
Difference between groups at 12 weeks (change scores 6–12 weeks) |
||
|
Mean (95% CI) |
p-value |
Mean (95% CI) |
p-value |
|
|
SPADI (0–100) |
1.8 (–5.7 to 9.2) |
0.64 |
0.4 (–5.1 to 6.0) |
0.88 |
|
SPADI adjusted |
3.6 (–2.8 to 10.0) |
0.27 |
0.4 (–5.1 to 6.0) |
0.88 |
|
Pain SPADI (0–100) |
–0.1 (–8.8 to 8.6) |
0.99 |
2.4 (–4.3 to 9.1) |
0.48 |
|
Function SPADI (0–100) |
3.6 (–3.7 to 10.9) |
0.34 |
–1.5 (–6.5 to 3.5) |
0.54 |
|
Pain (0–10) |
0.6 (–0.2 to 1.5) |
0.15 |
–0–4 (–1.1 to 0.2) |
0.20 |
|
GPSS (0–10) |
0.7 (–0.3 to 1.6) |
0.16 |
–0.8 (–1.6 to 0.0) |
0.05 |
|
SPADI: Shoulder Pain and Disability Index; GPSS: Generic Patient-Specific Scale; CI: confidence interval. |
||||
Patient’s Global Impression of Change and Patients’ Satisfaction with Treatment after 5 weeks. No significant difference could be found for either PGIC or PST at any follow-up (Table V). To test the robustness of the result for PGIC, the cut-off was changed from “slightly better” to “much better”, leading to a risk ratio (RR) of 1.05 (95% CI 0.68–1.64) after 5 weeks and 0.96 (0.66–1.39) at 12 weeks, respectively. High satisfaction was defined as a score of 8 or higher on the VNRS, and was more present in the intervention (87%) than in the control group (75%), but this difference was also not statistically significant (Table V).
|
Table V. Patients with a clinically important difference for every outcome measures at 5 weeks with relative risk (RR) (95% CI) |
||||
|
Outcomes |
Total group (n = 90) n (%) |
Intervention group (n = 46) n (%) |
Control group (n = 44) n (%) |
Relative risk (95% CI) |
|
Total SPADI score (> 10) |
51 (56.7) |
25 (54.4) |
26 (59.1) |
0.92 (0.64–1.32) |
|
GPSS (> 2) |
39 (43.3) |
21 (45.7) |
18 (40.9) |
1.12 (0.69–1.79) |
|
Mean Pain score (> 1) |
53 (58.9) |
32 (69.6) |
21 (47.7) |
1.46 (1.01–2.10) |
|
PGIC (Slightly and Much better) |
79 (87.8) |
42 (91.3) |
37 (84.1) |
1.06 (0.93–1.27) |
|
PGIC (Much better) |
42 (46.7) |
22 (47.8) |
20 (45.5) |
1.05 (0.68–1.64) |
|
PST (> 7) |
73 (81.1) |
40 (87.0) |
33 (75.0) |
1.16 (0.95–1.42) |
|
CI: confidence interval; SPADI: Shoulder Pain and Disability Index; GPSS: Generic Patient Specific Scale; PGIC: Patient’s Global Impression of Change; PST: Patients’ Satisfaction with Treatment. |
||||
Results for patients with a minimal clinically important difference. Looking at the absolute number of patients with a clinically significant change score in the outcome measures as defined a priori, no significant difference in any outcome could be found at any follow-up. A minor advantage for the intervention group was found for mean pain with a RR of 1.46 (95% CI 1.01–2.10), numbers needed to treat = 5 at 5, but not after 12, weeks. The 5-week results are shown in Table V.
Discussion
This randomized controlled trial investigated the short-term effect of individualized manual physiotherapy combined with an individualized exercise programme in comparison with individualized exercises alone on pain and functioning in patients with a clinical presentation of shoulder impingement syndrome. Both groups improved significantly in all outcome measures. A minor additional benefit of individualized manual physiotherapy could only be found for mean weekly pain at 5 weeks.
Baseline findings
Total SPADI score at baseline was similar to the scores found in other studies investigating shoulder complaints. However, the pain sub-score was markedly higher than the value for functional restriction, which became more obvious in the GPSS scores. This, together with small numbers of sick days, high activity levels, and low scores for fear avoidance and catastrophizing despite a mean duration of 105 weeks, would, in our opinion, be indicative for a dominant nociceptive pain mechanism at that time-frame in the course of the disorder. Table II summarizes the clinical baseline findings. Interestingly, all employed rotator cuff tests were negative in 89 cases. These tests do not have a discriminative ability in this population, and therefore we doubt their clinical usefulness in this patient group. However, over 80% of the total group showed translatory restrictions of the shoulder, and over 55% showed comparable signs of the cervical spine, which are definite indications for individualized manual therapy. We would have expected that the possibility to manually treat these contributing factors is an advantage and would contribute to a better physical improvement of the intervention group compared with the controls.
Influence of relevant covariates, additional medication and injection on Shoulder Pain and Disability Index
We identified mean pain, SPADI baseline scores, and the number of previous episodes as significant covariates. Combining them in a multivariable regression analysis for both follow-ups decreased the p-value for between-group differences to a certain degree, but did not change it to a significant level. In addition, the influence of medication or injection in our study on between-group results for the SPADI was not significant. For example, patients who received an injection in the first 5 weeks had a mean improvement of 10.7 (SD 12.4) on SPADI (total group 15.3 (SD 17.6)), which is in contrast to the results of Crawshaw et al. (31), who concluded that a combination of cortisone injection, manual therapy and exercises would lead to better short-term results than manual therapy and exercises alone.
Therapists’ expectations of outcome and obstacles with treatment application
Four out of 6 research therapists treating the intervention group believed in a better result for a combination of manual therapy and exercises in the short-term. Three out of 5 therapists from the control group, although blinded to the content of the IMPT and to all the examination results, favoured additional manual therapy over exercises alone. Reasons given for that were the ability to mobilize restricted joints, a more individualized care with a better placebo effect, the ability to address contributing factors individually and to give more precise instructions for the adaptation of daily activities. For the long-term prognosis 3 therapists from the intervention and 2 from the control group still favoured additional manual therapy. One control therapist expected a better result for exercises alone in the long-term. However, the results of this trial raise the question, to what degree the time-frame and the number of supervised sessions in the control group could be reduced without loss of effect.
Comparison with other studies
Few studies used the same exercise protocol as the basic intervention for both groups, on which the additional effect of individualized physiotherapy has been investigated. In the study of Bang & Deyle (32) 52 participants had 6 sessions of supervised flexibility and strengthening exercises. Only 2 out of 8 exercises were performed at home on a daily basis. The intervention group (n = 28) received manual physical therapy and specific home exercises to reinforce its effect. The intervention group showed better results for pain, strength and function after treatment and at 8 weeks. Conroy & Hayes (33) applied 9 sessions of hot-packs, soft-tissue mobilization, stretching, strengthening and pendulum exercises to 14 patients. Seven patients also received manual mobilization of the subacromial and glenohumeral joints. After treatment the manual therapy group had better results for pain, but not for function or range of motion. Senbursa et al. (34) randomized 77 participants to supervised exercises, supervised exercises combined with joint and soft-tissue mobilization, and to a group performing the exercises at home. There were no differences for pain, range of motion, strength, or the rate of positive tests after 4 and 12 weeks, except for function in favour of the manual therapy group at 4 weeks.
We also found a slightly better effect on pain in our intervention group at 5 weeks, but not for function or disability scores. The significant results of Conroy & Hayes (33) for pain improvement in favour of the intervention group may be an over-interpretation due to a type 1 error. Moreover, we do not support the statement of Senbursa et al. (34), that the “best results were seen in the manual therapy group” because both groups showed significant improvements with no statistically significant between-group differences. Interestingly, both of their exercise groups seemed to be equally effective. This raises the question about the number of supervised sessions needed, or, in this case, how supervision was done.
Differences between our results and the results of Bang & Deyle (32) can be explained by the differences in the exercises used. We started with a progressive non-provocative, pain-free and high-dose/low-resistance programme. In contrast, Bang & Deyle (32) used a low-dose programme and the chosen exercises, from our experience, were all highly pain provocative in SIS patients. This may have prevented progression and significant improvement in the exercise group of Bang & Deyle (32). Therefore, the results in favour of manual therapy may have rather been influenced by an ineffective exercise programme than by the manual therapy intervention itself.
Why did individualized manual physiotherapy not result in a stronger additional effect?
The additional effect of IMPT after 5 weeks is still questionable, even if a minor effect on pain level was detected, and may also be dependent on the quality of the applied exercise programme. Exercises in general show a comparable effect with (mixed) physiotherapy interventions (11, 35) and seem to be more effective than no intervention (13, 14, 36). The exercise protocol used in our study was designed to have a maximum effect. In addition to the shoulder joint muscles it also addressed the shoulder girdle muscles, posture, and mobility of the thoracic spine as important aspects (37–39). It was progressively organized with focus on a pain-free performance. Dosage was targeted to increase endurance and load capacity of the affected tissues and muscles, to achieve a maximum number of repetitions per training session, and an optimal dose-response relationship; the advantages of a high-dose compared with a low-dose exercise programme was shown by Osteras et al. (40). We also combined supervised and home exercises sessions supported by pictures and detailed written instructions. All these aspects may not have only improved effectiveness of the programme but seemed to reduce the possibility for IMPT to make a significant contribution. However, it might have accelerated improvement during the first 5 weeks, but this effect was lost after 12 weeks. One could also argue that the longer treatment time in the intervention group might also have influenced the effect on outcomes, but, if so, this did not significantly influence the results. The comparison of the number needed to treat to benefit for the intervention group (NNT = 21) with the NNT for the total group receiving IAEX (NNT = 2) for the first measurement point supports our assumption; that SIS, even if long-lasting or episodic, is a dominantly mechanical and nociceptive driven event.
Limitations
This trial was conducted in an outpatient physiotherapy setting under common conditions of the German health system. It provides detailed baseline information, well-described interventions, and results based on sufficient power, which allow the clinician to replicate and apply this information appropriately. We blinded therapists of the control group to all clinical information about their patients. Measurements of outcome were also blinded because therapists were not involved in this process. We could not blind patients, but they were kept naive to their allocation. The outcome measures we used were valid and easy to use in daily practice. The offer to participate in the study was solely based on clinical examination results without the use of diagnostic imaging, which reflected clinical practice and saved resources. Our experience was that patients, after being informed about the study, were willing to participate on this basis even if they had a recommendation for surgery from a general practitioner or orthopaedic surgeon. Because of ethical standards we did not include a placebo or passive control group in our study, so we could not estimate the contribution of a placebo effect to the results. However, results from other studies have shown that exercises are superior to a wait-and-see policy in the short-term (13, 14) and placebo treatment (41, 42). We involved only 6 outpatient physiotherapy clinics with 12 research therapists in our trial, so this may, to some degree, limit the external validity of the study results.
Conclusion
The results of this study show that individually adapted exercises are effective in the treatment of patients with SIS, and that IMPT had only a minor additional effect on pain intensity after 5 weeks. However, further research is necessary to confirm these results before definite recommendations can be made. Further research should also explore the components and parameters needed for an exercise programme to achieve maximum effect.
AcknowledgementS
We acknowledge the contributions of Cornelis Admiraal, Robert Blaser-Sziede, Isabella Knoecklein, Horst Baumgarten, Nils Jansen and their colleagues to this study as research therapists.
References
Appendix I. Additional intervention description |
Individually adapted exercises (IAEX) for both groups |
Procedure |
The “core programme” was instructed during the first 3–4 treatment sessions. |
Patients were monitored twice a week during their contact sessions. |
Frequency and dosage |
Patients performed the exercises twice a day for the first week, then once daily. |
Minimum exercises frequency during the week was 4, maximum 7. |
Dynamic exercises started with 2 sets of 10 repetitions and with low resistance (yellow rubber band). |
Shoulder and neck stretches were held for 10 s and repeated twice. |
Isometric scapular training positions were held for 10 s and repeated twice. |
Progression if patients performed the core programme without problems |
Sets were increased from 2 to 3. |
Repetitions (respectively seconds for the static exercises) were increased from 10 to 20. |
In a last step, resistance was increased from the yellow to the red and to the green rubber band. |
Exercises from an “additional programme” could be added if the patient could still perform the core programme without problems, whereas exercise C3 was replaced by exercise A4, C4 by A5, and C6 by A7. |
Patient instructions & stopping rules |
Patients were instructed on how to perform each single exercise. |
They received a booklet with pictures and descriptions of the exercises and the individually defined dosage. |
Patients had to stop an exercise if they had pain of more than 3 out of 10 on a VNRS during the exercises or longer than approximately 30 s after they had stopped an exercise. |
Patients recorded performance and difficulties with the programme in their log books which enabled the therapist to check the 24-h effect of the programme and to make adaptations. |
Therapists’ measures for adapting exercises to upcoming pain |
Reduction of resistance, sets, repetitions or the range of movement. |
If the total load of the programme was too provocative, patients were allowed to split the programme into 2 parts performing them at different times during the day. |
For some exercises an alternative version could be used (e.g. exercises C6b instead of C6a). |
If an exercise could not be performed due to pain, it was left out for the next 2 training sessions and was replaced by exercises AP1 and AP2. |
Contact time for the control group was 15–20 min. |
Intervention group: Individualized manual physiotherapy (IMPT) |
IMPT was based on clinical examination results and individual main complaints. |
Therapists were guided by a defined tripartite decision process to achieve a uniform and repeatable way of initial decision-making. |
Part 1 addressed signs that may predict a poor treatment outcome such as: |
≥ 3 episodes of shoulder pain in the last 12 months; pain > 5/10 on a VNRS. |
Duration of the current episode of > 6 weeks; signs indicating a rotator cuff tear. |
Restrictions of external rotation and/or elevation of the shoulder. |
Positive results led the therapist to focus initially on: |
Local manual pain treatment, pain-reducing exercises (AP1 and AP2), improving patients’ understanding about the pathology, and instructions for the most provocative ADLs to reduce pain events during the day. |
Behavioural instructions for painful ADLs and on manually assisted exercises to facilitate rotator cuff contraction. |
Manual mobilization of the identified restrictions. |
Part 2 summarized information about possible contributing factors such as general posture, patients’ main restricted activities identified through the GPSS, and other aggravating components, work place setting, leisure and sports activities. |
Ways of improving these factors and compensation strategies were then discussed. |
Part 3 defined the manual assessment of the glenohumeral and shoulder girdle joints, the cervical and upper thoracic spine. |
Initial treatment for positive findings: |
Painful and angular and/or translatory restricted peripheral joints were treated with manual glide techniques according to the concept of Kaltenborn (43). |
Comparable signs of the spine segments were treated with posterior-anterior glides or coupled movements. |
Shortened muscles were stretched according to the description of Evjenth & Hamberg (44). |
Neural tissue was treated according to Butler (45). |
Dosage for interventions of part 3: |
Treatment intensity was limited by pain of > 4/10. |
Initial duration of the glide techniques and the stretches was 20–30 s. Further dosage was based on reassessment results. |
Subsequent treatment decisions were made with the help of an adapted clinical reassessment process based on the test-retest principle described by Maitland (46). |
In addition to the general information in the shoulder booklet, this group received detailed information about the assessment results and therapy interventions. |
Contact time for the intervention group was 20–30 min. |
VNRS: visual numeric rating scale; ADL: activities of daily living; GPSS: Generic Patient-Specific Scale. |
Appendix II. Restricted activities from the Generic Patient-Specific Scale | |
Activity | Frequency, n |
Activities in an upward direction | 89 |
Reaching overhead/upwards | 18 |
Working overhead | 19 |
Lifting above shoulder height | 17 |
Drying/combing or washing hair | 11 |
Getting something down from a cupboard | 9 |
Holding something in front of the body | 15 |
Lying on the affected shoulder | 33 |
Sports activities | 24 |
Playing tennis | 2 |
Swimming | 2 |
Fitness training | 4 |
Other | 16 |
Getting dressed | 23 |
Putting on a jacket | 6 |
Pushing forward with the affected arm | 21 |
Cleaning windows | 7 |
Housework | 16 |
Activities with hand behind back | 16 |
Steering a car | 12 |
Carrying | 10 |
Computer work (with/without a mouse) | 10 |
Body care | 5 |
Resting on/pushing downwards | 5 |
To buckle up in the car | 3 |
Other | 3 |
Total | 270 |
