Cecilie Røe, MD, PhD1,2, Toril Skandsen, MD, PhD3,4, Audny Anke, MD, PhD6,7, Tiina Ader, MD12, Anne Vik, MD, PhD3,5, Stine Borgen Lund, MSc5, Unn Manskow, MPH6,7, Snorre Sollid, MD, PhD8, Terje Sundstrøm, MD, PhD9,10,11, Morten Hestnes, RN13 and Nada Andelic, MD, PhD1
From the 1Department of Physical Medicine and Rehabilitation, Oslo University Hospital, Ulleval, 2Faculty of Medicine, University of Oslo, Oslo, 3Department of Neuroscience, Faculty of Medicine, Norwegian University of Science and Technology (NTNU), 4Department of Physical Medicine and Rehabilitation and 5Department of Neurosurgery, St Olavs Hospital, Trondheim University Hospital, Trondheim, 6Department of Rehabilitation, University Hospital of North Norway, 7Faculty of Health Sciences, Institute of Clinical Medicine, University of Tromsø, 8Department of Neurosurgery, University Hospital of North Norway, Tromsø, 9Department of Neurosurgery, Haukeland University Hospital, 10Department of Biomedicine and 11Department of Surgical Sciences, University of Bergen, 12Department of Physical Medicine and Rehabilitation, Haukeland University Hospital, Bergen and 13Trauma Registry, Oslo University Hospital, Ulleval, Oslo, Norway
OBJECTIVE: The aim of this study was to investigate the influence of age on mortality and 3-month outcome in a Norwegian cohort of patients with severe traumatic brain injury (TBI).
METHODS: Norwegian residents ≥ 16 years of age who were admitted with a severe TBI to the country’s 4 major trauma centres in 2009 and 2010 were included, as were adults (16–64 years) and elderly patients (≥ 65 years).
RESULTS: Half of the adult subjects and 84% of the elderly subjects were injured by falls. One-third of the adults and half of the elderly subjects were admitted to a local hospital before being transported to a regional trauma hospital. Subdural haematomas were more frequent in the elderly subjects. One-quarter of adults and two-thirds of the elderly subjects died within 3 months. At 3 months, 41% of the adult survivors were still in-patients, mainly in rehabilitation units (92%). Of the surviving elderly subjects, 14% were in-patients and none were in rehabilitation units. There was no difference in functional level for survivors at the 3-month follow-up.
CONCLUSION: Old age is associated with fall-induced severe TBI and high mortality rates. Less intensive treatment strategies were applied to elderly patients in the present study despite high rates of haemorrhage. Few surviving elderly patients received rehabilitation at 3 months post-injury.
Key words: traumatic brain injury; aged; treatment; outcome; prognosis.
J Rehabil Med 2013; 45: 734–740
Correspondence address: Cecilie Røe, Department of Physical Medicine and Rehabilitation, Oslo University Hospital, Ulleval, Oslo, Norway. E-mail: e.c.t.roe@medisin.uio.no
Accepted April 23, 2013
INTRODUCTION
Traumatic brain injury (TBI) is recognized as a major health problem, with 15% of the patients with TBI admitted to trauma centres having severe TBI (1). These patients require intensive medical care and long-term rehabilitation (2). Mortality after TBI is higher than for other injuries and is nearly 30% for severe TBI (3). Some patients with severe TBI develop long-standing deficits that interfere with independent living, reduced levels of functioning and restrictions on activities (4). Several factors are associated with mortality and unfavourable outcome, with age and injury severity being the dominating determinants (5, 6).
Cardiac co-morbidity and coagulopathy are well-known risk factors that significantly increase overall mortality in elderly patients with TBI (7). With increasing age, autoregulatory capacity decreases, resulting in diminished cerebrovascular control (8). Moreover, as indicated by animal studies, there is prolonged acute oedema, increased permeability of the blood–brain barrier and increased neurodegeneration in the ageing brain following injury (9).
Evidence also suggests that elderly people are treated less aggressively than younger people (10), and that it would be beneficial to increase the treatment intensity for this large group of patients (11). However, treatment choices can be more difficult when treating elderly patients. An unconscious state may be interpreted by emergency staff as the result of a cardiovascular episode rather than a TBI. Treatment strategies may be influenced by the fear that rescuing elderly patients from death may result in a vegetative or very low functional status (12). To provide sound management guidelines for elderly patients, there is a need for more knowledge about the impact of age on injury characteristics and treatment choices.
The aim of this study was therefore to investigate the influence of age on mortality and 3-month outcome in a Norwegian cohort of patients with severe TBI.
MATERIAL AND METHODS
Design and study region
This project was a prospective, multicentre, cohort study, comprising patients admitted with severe TBI to the regional hospitals in all 4 health regions in Norway. Norway has a land area of 323,758 km2 and an adult population (aged >16 years) of 3.8 million (Statistics Norway). There is a public, 3-level hospital structure, with local hospitals serving small areas, central hospitals serving larger areas (counties) and a total of 5 university hospitals serving these hospitals in a regional manner.
Inclusion
Norwegian residents ≥ 16 years of age who were admitted to their regional trauma centres within 72 h of a severe TBI were considered for inclusion in this study. Severe TBI was defined by International Classification of Diseases – 10th revision (ICD 10) criteria (S06.1–S06.9) and a Glasgow Coma Scale (GCS) score between 3 and 8 within the first 24 h after injury. The regional trauma centres were the University Hospital of North Norway for the northern region, St Olav’s Hospital Trondheim University Hospital for the middle region, and Oslo University Hospital for the south-eastern region. In the western part of the country, patients are equally distributed between Haukeland University Hospital and Stavanger University Hospital. Unfortunately, Stavanger University Hospital was not able to participate. Exclusion criteria were chronic subdural haematomas (SDH), pre-injury cognitive disability, and severe psychiatric disease or drug abuse. This study was approved by the regional Committee for Medical Research Ethics, South-East Norway.
During the study period (January 2009 to January 2011), 276 patients were eligible for inclusion: 42 in the northern region, 40 in the middle, 16 in the west, and 178 in the south-east. Five patients did not consent to participate in the interviews at 3-month follow-up (4 from the south-east and 1 from the north) and were omitted from the analysis. Hence, 271 patients were included (Fig. 1).
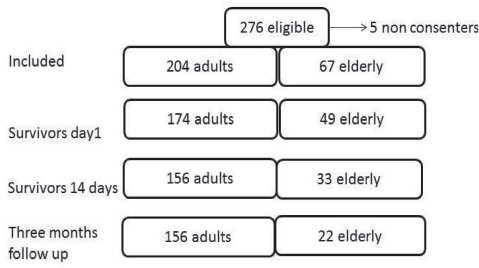
Fig. 1. Included and 3-month surviving adults and elderly patients.
Data collection
Data registration was based on a systematic review of hospital journals (paper and electronic records) and data from the trauma registries in the west and south-east. Trauma scores from the northern and middle regions were calculated by certified professionals. Supplementary information regarding demographic data and functional levels was collected from relatives of the patients or, preferably, from the patients themselves using a standardized telephone interview 3 months after the injury occurred.
Demographic and injury characteristics
The subjects were classified as adults (16–64 years) or elderly subjects (≥ 65 years), a dichotomization commonly employed for developed countries (www.who.int/healthinfo/survey/ageingdefnolder/en/index.html). The ICD-10 diagnoses of comorbid conditions were recorded and categorized anticoagulant status was defined by the use of warfarin or platelet inhibitors. The influence of alcohol or other substances at admission was categorized as yes or no, based on clinical judgement and blood or urine analysis, when available. Transport time from accident scene to the initial hospital was recorded. Intermediate stays at local hospitals prior to admittance to the trauma centre was recorded as yes or no.
Injury-related variables
The GCS score was assessed at the accident scene and at hospital admittance; we recorded the lowest GCS score recorded within the first 24 h. The duration of post-traumatic amnesia (PTA) was categorized as < 7, 7–13, 14–20, 21–27 or > 27 days (13). The Injury Severity Score (ISS) version 2008 was applied to indicate overall trauma severity.
The computed tomography (CT) findings were described according to the presence of contusions and haemorrhages (epidural, subdural and subarachnoid). These findings were also categorized according to the Rotterdam CT classification. The Rotterdam CT score is based on the compression of basal cisterns, midline shift, epidural mass lesion and intraventricular blood or subarachnoid hemorrhage and is scored from 1 (least severe) to 6 (most severe). The scan showing the greatest injury severity was used for scoring. The Rotterdam CT scores were interpreted by one neuroradiologist at each trauma centre for the northern and south-eastern regions, and a neurosurgeon for the western and middle regions.
Medical complications and interventions
Hypoxia was defined as at least one episode of oxygen saturation (SaO2) < 90% before or after admittance to a hospital. Hypotension was defined as at least 1 episode of systolic blood pressure (BP) < 90 mmHg before or after admittance to a hospital. Cerebral perfusion pressure (CPP) was classified as reduced when it was <60 mmHg; an intracranial pressure (ICP) of > 30 mmHg was categorized as elevated; and pyrexia was defined as 1 or more recordings of a body temperature (temp) of > 38ºC. The ICD-10 diagnoses of medical complications were recorded and categorized for analytical purposes as present or absent. Patients who received any type of surgery, including ICP monitoring, cerebrospinal fluid (CSF drainage), craniotomy and craniectomy were registered as yes and others as no. The administration of mannitol, hypertonic saline, vasopressors, anti-epileptics or antipsychotics was also recorded as yes or no. The number of days on a respirator and number of days with active sedation were recorded. Treatment with tracheostomy and percutaneous endoscopic gastrostomy (PEG) was similarly dichotomized.
Early outcome
We registered all deaths within the first 3 months after injury. Residency at 3 months was categorized as “at home” or “not at home” (hospital, rehabilitation units, or nursing homes). The global functional outcome at 3 months was evaluated in survivors using a structured interview with the Glasgow Outcome Scale Extended (GOSE) (14).
Data analysis and statistics
Data are presented as the mean value with SD or the median value with 95% confidence intervals (CI) and range for skewed data. The χ2 test for contingency tables was used to detect associations between categorical independent variables. Age was dichotomized into adults (16–64 years) and elderly subjects (≥ 65 years) for these analyses. Percentages or odds ratios (OR) and CI are presented for dichotomized variables across the age categories. Independent t-tests or the Mann-Whitney U test were applied to compare normally distributed and skewed values, respectively, in adults and elderly people.
Binary logistic regression was applied to investigate the effect of age (adult = 0/elderly = 1) on intubation (yes = 0/no = 1) at the accident scene, controlling for the GCS score at the accident scene (a log-transformed score value due to skewed distribution) and the injury mechanism (fall = 0, other injuries = 1).
Binary logistic regression analysis was applied to examine the effect of age on mortality (0 = dead, 1 = survival at 3 months), residence (0 = hospital, 1 = home) and GOSE score at 3 months. GOSE scores were dichotomized into unfavourable outcome (vegetative state or severe disability = 0) and favourable outcome (moderate disability or good recovery = 1). The independent variables were the GCS score (3–8), pupillary dilation (no = 0, yes = 1) and Rotterdam CT score (1–6), in addition to age.
Subsequently, we added comorbidity (no = 0, yes = 1), anticoagulant status (warfarin, platelet inhibitors) (no = 0, yes = 1) and intubation, hyperosmolar therapy and vasopressor medication (no = 0, yes = 1) and intracranial surgery (no = 0, yes = 1) to all models.
Adjusted OR with 95% CI were calculated using the highest values as references. Cox and Snell and Nagelkerke R squares are given. The possible multicollinearity of the independent variables was examined. The present sample size could capture a twice as high odds of mortality in the elderly group compared with the adult group, with a power of 90%.
A significance level of 5% was used. Statistical analyses were performed with SPSS 19.0 and IBM Sample Power 3 (SPSS Inc., Chicago, IL, USA).
RESULTS
Pre-injury characteristics
Three subjects lived in sheltered homes at the time of injury (two in the elderly group). Thirty-nine percent of adults and 58% of the elderly subjects were married or cohabitant (Table I). In addition, 15% of the adults and 3% of the elderly group were living with adults other than their spouses. Four percent of the elderly group were still working, whereas 60% of the adult subjects were working. Comorbidity was more common in the elderly subjects. Only 12% of adults had multiple diseases, whereas nearly half of the elderly subjects had several comorbid conditions. Half of the adults (46%) and 90% of the elderly subjects had comorbid disorders. No single condition was predominant among the adult group, whereas cardiovascular disorders dominated among the elderly subjects.
Injury mechanism and transportation to hospital
Fall is the leading cause of injury, with the highest frequency among elderly subjects (Table I). Forty percent of the injured patients were admitted to a local hospital prior to transport to a regional trauma hospital. The elderly subjects were significantly more frequently transported to a local hospital prior to transfer to the trauma centre (Table I). Even when injured in traffic accidents, 5 out of 8 elderly subjects were transported to the local hospital. The median time of transport to the first hospital was 60 min, ranging from 6 min to nearly 11 h, regardless of the injury mechanisms (p = 0.48) or age (p = 0.56).
|
Table I. Demographic characteristics and injury mechanisms of adult and elderly subjects |
||||
|
Adult subjects (16–64 years) (n = 204) % (n) |
Elderly subjects (≥ 65 years) (n = 67) % (n) |
χ2 |
p-value |
|
|
Male |
82 (168) |
63 (n = 42) |
11.04 |
0.01 |
|
Married/cohabitant |
39 (80) |
58 (n = 39) |
7.48 |
0.006 |
|
Comorbidity |
45 (91) |
90 (n = 60) |
25.71 |
< 0.001 |
|
Anticoagulant medicationb |
6 (12) |
60 (n = 40) |
91.81 |
< 0.001 |
|
Injury mechanism Fall Transport Violence Sports/other |
49 (99) 39 (80) 7 (14) 5 (11) |
84 (56) 12 (8) 1 (1) 3 (2) |
40.61 |
< 0.001 |
|
Transport via local hospital |
34 (70) |
52 (35) |
6.68 |
0.01 |
|
Substance influencea |
38 (77) |
16 (11) |
10.80 |
0.005 |
|
aClinical evaluation or results of blood test documented in medical record. bAnticoagulation and platelet inhibitors. |
||||
Injury severity and complications
GCS score at the accident scene was significantly higher in older patients, while no difference was found between the age groups for the lowest GCS score within 24 h (Table II). Pupillary dilation was observed in the pre-hospital phase in 41% of adults and 33% of the elderly subjects (χ2 = 0.30, p = 0.59), and dilation was noted at admittance or during the hospital stay in 41% of adults and 42% of elderly subjects (χ2 = 0.05, p = 0.82).
The elderly subjects had significantly higher Rotterdam CT scores and significantly lower ISS scores (Table II). The type of intracranial lesions was equally distributed across age groups (Fig. 2), but the elderly subjects had an OR of 3.25 (CI 1.67–6.33, χ2 = 12.80, p < 0.001) for SDH compared with adults. Hypoxia and hypotension were frequent, with no differences between the age groups (Table III). These patients also had a wide variety of complications in general, which were of respiratory, cardiovascular, metabolic, hormonal and infectious origin, and no statistically significant differences between the groups in the overall frequency of these complications were observed (Table III).
|
Table II. Injury severity in adult and elderly subjects. Proportion of patients with pupil dilation reported either pre- or during hospital stay |
|||
|
Adult subjects (16–64 years) (n = 204) % (n) |
Elderly subjects (≥ 65 years) (n = 67) % (n) |
p-value |
|
|
GCS score accident scene, median (range) |
6 (3–15) |
8 (3–15) |
< 0.001 |
|
GCS score lowest, median (range) |
5 (3–8) |
5 (3–8) |
0.54 |
|
Pupil dilation, % |
41 |
45 |
0.82** |
|
AIS head, median (range) |
5 (2–5) |
5 (2–5) |
0.38 |
|
ISS, median (range) |
28 (4–75) |
25 (10–59) |
0.02* |
|
Rotterdam CT score, median (range) |
4 (1–6) |
5 (2–6) |
< 0.001 |
|
p-values from independent sample t-test and from Mann-Whitney U test when the distribution was skewed (*), and from χ2 test for the presence or absence of pupillary dilation either before or during hospitalization (**) . GCS: Glasgow Coma Scale; AIS: American Spinal Injury Association Impairment Scale; ISS: Injury Severety Score; CT: computed tomography. |
|||
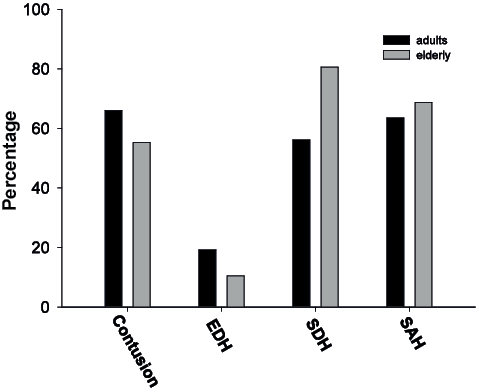
Fig. 2. Intracranial injuries in adults and elderly subjects expressed as the percentage of each age group: adults (n = 204) (black bars), elderly subjects (n = 67) (grey bars) (*p < 0.05). EDH: epidural haematomas; SDH: subdural haematomas; SAH: subarachnoid hemorrhage.
|
Table III. Medical complications in adult and elderly subjects |
||||
|
Adults (16–64 years) (n = 204) % (n) |
Elderly (≥ 65 years) (n = 67) % (n) |
χ2 |
p-value |
|
|
Hypoxia (SaO2 < 90%) |
51 (104) |
39 (26) |
0.001 |
0.47 |
|
Hypotension (BP < 90 mmHg) |
48 (97) |
48 (32) |
0.53 |
0.97 |
|
ICP/CPP not monitored |
27 (55) |
60 (40) |
23.74 |
< 0.001 |
|
ICP > 30 mmHg |
32 (65) |
12 (8) |
1.84 |
0.21a |
|
CPP< 60 mmHg |
33 (67) |
18 (12) |
0.15 |
0.56a |
|
Pyrexia (temperature > 38ºC) |
53 (109) |
42 (28) |
2.66 |
0.27 |
|
Other complications |
66 (156) |
81 (54) |
0.43 |
0.51 |
|
aComparison among adults and elderly receiving ICP/CPP monitoring. BP: blood pressure; CPP: cerebral perfusion pressure; ICP: intracranial pressure. |
||||
Interventions
Fifty percent of the adult patients were intubated at the accident scene, compared with 18% of elderly subjects (χ2 = 20.99, p < 0.001, OR 0.22, CI 0.11–0.44). In a logistic regression controlling for GCS score and the injury mechanism, age remained a significant predictor of non-intubation at the accident scene (OR 0.32, CI 0.15–0.69, p = 0.003).
Medication as well as monitoring of ICP varied between the adult and elderly subjects (Tables III and IV).
|
Table IV. Medical and surgical interventions in adults and elderly receiving vasopressor and osmotic medication, Cerebrospinal fluid (CSF) drainage, and intracranial surgery. χ2 test and p-values for the difference between adult and elderly subjects |
||||
|
Adult subjects (16–64 years) (n = 204) % (n) |
Elderly subjects (≥ 65 years) (n = 67) % (n) |
χ2 |
p-value |
|
|
Vasopressor medication |
77 (156) |
55 (37) |
11.05 |
0.001 |
|
Hyperosmolar therapy |
38 (77) |
25 (17) |
3.33 |
0.08 |
|
CSF drainage |
18 (37) |
10 (7) |
2.00 |
0.16 |
|
Craniotomy |
23 (47) |
30 (20) |
1.41 |
0.24 |
|
Craniectomy |
14 (28) |
2 (1) |
7.95 |
0.005 |
ICP and CPP were not monitored in 60% of the elderly subjects and 27% of the adults (Table III). Among those surviving at 3 months, 64% of elderly subjects and 88% of adult subjects had received ICP monitoring in the acute phase (χ2 = 3.70, p = 0.05). In patients with ICP monitoring there were no statistically significant difference in the percentage of patients with ICP values > 30 mmHg in the two age groups (Table III).
The 178 surviving patients spent a mean of 10 (SD 12) days on a respirator and were sedated for a mean of 6 (SD 6) days, regardless of age group (p > 0.36).
The percentage receiving tracheostomy or PEG was not different between the two age groups (χ2 = 1.03, p = 0.31 and χ2 = 0.08, p = 0.77), but the number of elderly subjects included in these analyses was low.
Mortality
The overall 3 months mortality was 34%, corresponding to 24% in the adult group and 67% in the elderly group. Eighty-six percent of the deaths occurred within 2 weeks (Fig 1) and the median time from the accident to death was within 1 day in the adult subjects and 2 days in the elderly subjects.
There was significantly higher mortality in the elderly subjects after adjusting for injury severity (GCS score, Rotterdam score and pupillary dilation) (Table V). Comorbidity and treatment factors did not contribute to the model or change the effect of age.
|
Table V. Binary logistic regression analysis exploring the effect of age on survival (all patients), home residence and favourable outcome (Glasgow Outcome Scale Extended) (survivors at 3-month follow-up). The independent variables were Glasgow Coma Scale (GCS) (3–8), pupillary dilation (yes/no) and Rotterdam score (1–6), in addition to age (adult/elderly). Adjusted odds ratios (ORs) with 95% confidence intervals (95% CI) and Cox & Snell and Nagelkerke R squares are given |
||||
|
Model |
OR |
95% CI |
p-value |
R2 Nagelkerke (Cox and Snell) |
|
Survival (n = 271) GCS score Pupil dilation Rotterdam Age |
1. 57 0.57 0.28 0.09 |
1.27–1.95 0.27–1.19 0.18–0.43 0.04–0.21 |
<0.001 0.13 <0.001 <0.001 |
0.59 (0.42) |
|
Home residence (n = 178) |
0.22 (0.16) |
|||
|
GCS Pupil dilation Rotterdam Age |
1.17 0.41 0.64 2.18 |
0.98–1.39 0.21–0.81 0.45–0.92 0.79–6.03 |
0.09 0.01 0.01 0.13 |
|
|
Favorable outcome (n = 178) |
0.11 (0.08) |
|||
|
GCS Pupil dilation Rotterdam Age |
1.25 0.67 0.79 0.46 |
1.04–1.52 0.33–1.37 0.54–1.13 0.17–1.22 |
0.02 0.27 0.20 0.39 |
|
Three-month outcome
At 3 months, 41% of the adult survivors were still in-patients, mainly in rehabilitation units (92%). Of the surviving elderly subjects, 14% were in-patients and none were in rehabilitation units. Six percent of adult subjects and 18% of elderly subjects stayed in nursing homes or adapted living units. Thus, 53% of adult subjects and 68% of elderly subjects had returned to their own home. For survivors, the mean GOSE score at 3-month follow-up was 5.00 (SD 1.52), and statistically significant differences in distribution between the age groups were not observed (χ2 = 11.43, p = 0.08) (Fig. 3). Elderly subjects were more likely to be discharged to their home at 3 months after adjusting for injury severity (GCS score, Rotterdam score and pupillary dilation) (Table V); the logistic regression with dichotomized GOSE scores indicated a tendency toward more unfavourable outcomes for the elderly subjects, although the results did not reach statistical significance. Low level of comorbidity was a significant predictor of favourable outcome (p = 0.03), but did not add unique explanatory value to the model (R2 Nagelkerke = 0.42), whereas intubation, medication and surgery did not contribute to the models or change the effect of age.
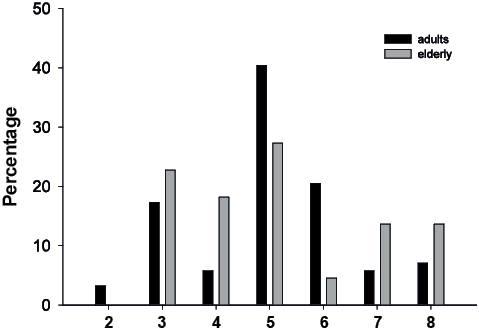
Fig. 3. Distribution of Glasgow Outcome Scale Extended (GOSE) score expressed as the percentage of survivors in each age group: adults (n = 156) (black bars) and elderly subjects (n = 22) (grey bars).
DISCUSSION
The present study adds to the huge amount of evidence of TBI associated with high mortality in elderly subjects. In the present study, mortality was also higher in elderly subjects after adjusting for injury severity. Elderly subjects had a higher burden of comorbidity, but the treatment also differed between the two age groups. Elderly subjects were less frequently intubated and more often admitted to care via local hospitals. ICP and CPP were measured in less than half of the elderly subjects. At 3 months, two-thirds of elderly subjects had returned to their homes, whereas a larger number of adult subjects remained in hospital, mainly due to sustained rehabilitation. Injury severity, as evaluated by GCS, was the single best predictor of unfavourable outcome, and more so in elderly subjects.
Falls are the main cause of TBI in elderly subjects (15). Several actions are effective in fall prevention, including better control of medication, adapted environments, adjustments for reduced vision and hearing, as well as activity and exercises, even though ageing and comorbidity itself may not be prevented (16). Alcohol may also be less well tolerated in elderly subjects, and result in falls, although the overall percentage of injuries associated with alcohol was lower in elderly subjects than adult subjects in the present study. Hence, attention should be paid to the prevention of falls, including better monitoring of medication and interventions to improve mobility and balance (17).
The present study also shows that elderly subjects were more often transported to a local hospital, which is a negative predictor of outcome (18, 19). The high number of falls resulting in unconsciousness without information on the trauma mechanisms may lead to a suspicion of a cardiovascular disorder or other medical conditions. However, the lower rate of intubation in patients with reduced levels of consciousness is less likely to be influenced by such factors. Thus, there may be potential for improvement in the pre-hospital treatment of elderly subjects, possibly with improved survival rates for these patients.
The elderly group had higher GCS scores at the accident scene, whereas pupillary dilation in the pre-hospital phase was equally frequent in both age groups. However, the worst GCS score within 24 h was similar across age groups. The elderly subjects had slightly more extensive intracranial injuries as evaluated by the Rotterdam score. These differences may be partially explained by more frequent SDHs in the elderly subjects, with gradually developing haemorrhages causing clinical deterioration as well as intracranial findings on CT (20, 21). Improved diagnostic evaluation and direct and swift transport to a trauma centre may improve the prognosis in elderly patients (19).
In hospitals, a case fatality rate between 20% and 40% for severe TBI is reported in most European countries (22). TBI is still the major cause of death and disability in young adults in developed countries (23). However, a 50% reduction in the mortality rate due to severe TBI over the last 150 years has been reported (24). Safer cars and roads and improved pre-hospital and emergency management have contributed to this trend (3, 25). However, in Western countries in the most recent decades, there is an increasing incidence of falls among elderly citizens (16), resulting in severe TBI (26) that are associated with poor outcomes (27, 28). Similar results have emerged from the Nordic countries, with increases in the mortality rates for elderly people, particularly women, and an increase in the mean age of TBI casualties from 45 to 53 years of age for men and 54 to 65 years of age for women (29). Although the overall mortality was approximately 30% in the present study, only one-third of the present patients ≥ 64 years of age survived.
The relationship between age and probability of death is a subject of debate, with some studies showing a linear association and other studies showing an association only in patients > 40 years of age (30, 31). However, the marked shift in injury mechanisms is a feature of patients aged above 60–65 years, with a steep increase in the incidence of falls (32). The influence of age on treatment choices, controlling for all other injury variables, is a difficult topic. The increased morbidity associated with aggressive management of TBI in some studies has been interpreted as support for a more conservative treatment policy in the elderly status (12). However, recent studies clearly show an overall benefit of aggressive treatment in older patients (11), and even the most severely injured elderly people may recover (33). Higher mortality in elderly people may have influenced the recorded rate of osmotic and vasopressor medication, ICP and CSF drainage in the present study. However, a clear tendency to lower rate of ICP monitoring in elderly people was also found among the surviving patients. It is worth noting that less aggressive monitoring and treatment may also cause higher mortality (11). The major improvement in outcome over the last years reflects the use of protocols to guide all phases of treatment for these patients, with focus on certain groups now recognized as being at greater risk, in particular elderly people, and anti-coagulated patients (34). Thus, the lack of national guidelines for hospital treatment of severe TBI in Norway adapted to elderly people is a major challenge.
The high number of elderly people receiving anti-coagulation and anti-thrombotic medications is worth noting. Although they did not influence the mortality or crude measurements of early outcome in the present study, these medications may contribute to minor falls that result in severe TBI, and interact with complications and treatment results (34). Anti-thrombotic medication may, in particular, contribute to the high frequency of SDH in elderly people, where prompt intervention and cranio- tomy are of particular importance (35). The lack of significant differences in craniotomy frequency between adults and elderly people in the present study may therefore indicate a less aggressive treatment approach towards the elderly population.
In this study, the GOSE scale was dichotomized between severe and moderate disability, according to the customary procedures in the literature (36). However, recent evidence indicates that the application of the GOSE as an ordinal dependent variable markedly increases the sensitivity of the analysis (37). However, with only 22 surviving patients in the elderly group, this method was not applied in the present study. Outcome measurement focusing in more detail on activities and participation tasks would probably be more sensitive, but also more difficult to apply comparing patients still in rehabilitation units with patients discharged to their homes (38). This multicentre study design did not allow for a more detailed registration of pre-injury morbidity and functional status. Such information, if available, could also have contributed to a more sensitive prediction of outcomes (39).
The modified predictive factors from the impact study (38) are well adapted to describe mortality in the present study, and additional injury variables and treatments did not contribute significantly to the results. However, these models performed more poorly in predicting functional outcome at 3-month follow-up when only surviving patients were included.
The relatively low proportion of elderly subjects in nursing homes and the high proportion residing at home 3 months post-injury is worth noting. This represents a challenge to the healthcare system in municipalities as well as to families and other caregivers. In contrast, nearly 40% of the adults were still receiving rehabilitation at this time point. Given the information regarding rehabilitation in elderly stroke patients, the importance of placing elderly patients with TBI in rehabilitation units should be reconsidered.
Although this study included all severe TBIs in Norway (except for Stavanger County) over a 2-year period, the number of patients surviving and eligible for follow-up in each age group was relatively low. The strength of the study is that it used a representative cohort because all severe trauma patients in Norway that were admitted to regional trauma referral centres are referred to further care in public hospitals or rehabilitation units (40). Despite this, a multicentre study will always be flawed by differences between study centres that are not documented and by biases in registration procedures. The present study was based on registrations of crude measurements and procedures. For example, a more detailed monitoring of ICP also covering levels between 20 and 30 mmHg would be preferred. Our results may also be influenced by the choice of cut-off between adult and elderly subjects at 65 years. Subgrouping the elderly subjects further was difficult in the present study as there was a low number of elderly survivors. This limited number of surviving elderly subjects also flaws the ability of the study to detect differences in outcome. The power of the study to capture the large age-related differences in mortality was high. However, the present differences in favourable outcome between the age groups render the power for these analyses down to 30%.
By studying patients admitted to the trauma referral centres, patients dying in the pre-hospital phase or at the local hospital were not included. Hence, overall mortality from severe TBI cannot be assessed in the present study, and may possibly exaggerate the age differences.
In conclusion, old age is associated with fall-induced severe TBI and high mortality rates. Less intensive treatment strategies were applied to elderly patients in the present study despite high rates of haemorrhages. Few surviving elderly patients received rehabilitation at 3 months post-injury. Recent evidence suggests that this patient group would benefit from a more intensive treatment strategy, and guidelines are needed for this purpose.
ACKNOWLEDGEMENTS
The authors would like to thank the Trauma Registry at Oslo University Hospital, Ulleval and Haukeland University Hospital for providing data regarding injury severity. We also thank Cand. Med. Tone Jerstad for her effort in analysing the majority of CT scans and providing the Rotterdam scores.
References
