Isabelle V. Bonan, PhD1,2, Florence Gaillard1, Sophie Tasseel Ponche, MD4, Adelaïde Marquer, MD2, Pierre P. Vidal, PhD2,1 and Alain P. Yelnik, PhD2,4
From the 1PRM Department, University Hospital, Faculty of Medicine, University of Rennes, Rennes, 2COGNition and ACtion Group, Université Paris Descartes – CNRS – UMR, 3Department of Life Sciences, University of Paris Diderot, Sorbonne Paris Cité and 4PRM Department GH Saint Louis Lariboisière F. Widal AP-HP, Université Paris Diderot, Paris, France
BACKGROUND: Shortly after stroke, patients exhibit excessive sensitivity to visual, proprioceptive and vestibular perturbations regarding balance control.
OBJECTIVE: To evaluate the stability of this perceptual behaviour after stroke and test the relationships between sensory sensitivity and balance.
METHODS: Thirty subjects following a hemispheric stroke (mean age 54.7 (standard deviation (SD) 10.6 years), 21 men, right hemisphere lesion = 13) and 30 control subjects (mean age 52.0 (SD 12.0), 14 men). Sensitivity to sensory perturbations was evaluated using the displacement of the centre of pressure during tendon vibration (proprioception score), optokinetic (visual score) and galvanic perturbations (vestibular score) while standing on a force-platform a mean of 2 months after stroke, and 1 month later. Balance and independence were evaluated using the Berg Balance Scale (BBS), Timed Up and Go test (TUG) and Barthel Index (BI).
RESULTS: Global sensitivity to perturbations decreased (p = 0.001). Patients remained more sensitive to visual perturbation than did controls (p = 0.033). The Vestibular Score was correlated with BBS (Rs = –0.576, p = 0.006), TUG (Rs = 0.408, p = 0.045), BI (Rs = –0.481, p = 0.016); the Visual Score was correlated with BBS (Rs = –0.500, p = 0.019), TUG (Rs = 0.401, p = 0.049).
CONCLUSION: The initial months following stroke appear to be a period of individual perceptual motor adaptation. Sensory re-weighting is likely to be a major component of that process.
Key words: postural balance; stroke; sensory function; hemiplegia.
J Rehabil Med 2015; 47: 00–00
Correspondence address: Isabelle Bonan, Physical and Rehabilitation Medicine Department, Centre Hospitalier Universitaire, 2 rue Henri le Guilloux 35000 Rennes, France. E-mail: isabelle.bonan@chu-rennes.fr
Accepted Feb 18, 2015; Epub ahead of print Apr 21, 2015
INTRODUCTION
After stroke, recovery of balance is of crucial importance in order to achieve autonomy in activities of daily living (1, 2). Balance control requires, in addition to adequate motor control, the integration of visual, vestibular and somatosensory inputs (3, 4). The sequence of motor recovery is relatively clear; it follows a non-linear logarithmic pattern, with most improvements occurring during the first 15 weeks post-stroke, regardless of the severity of the initial motor deficit (5, 6). It is also now common to define residual motor impairments as stable and chronic 6 months post-stroke (7). On the other hand, less is known about the time course of perceptual recovery. Most stroke patients are excessively reliant on visual information to control their posture in both the frontal and sagittal planes (8–12). This excessive reliance on visual information is present shortly after stroke as well as later post-stroke (8, 13, 14). In a recent study we showed that, soon after stroke, the postural reactions of patients to visual, but also vestibular and proprioceptive, perturbations were initially more pronounced than for control subjects (15). However, in contrast with what might be expected, the increased visual reliance in stroke patients does not necessarily entail neglect of vestibular and proprioceptive information. In addition, similarly to healthy subjects, a large degree of inter-individual variability was found in hemiparetic subjects in response to sensory perturbations.
Similarly to motor recovery, there may be different sequences in perceptual recovery (16, 17). The aims of the present study were to evaluate the time course of recovery of sensitivity to perceptual perturbations in hemiparetic patients with regard to balance during the first months following stroke, using the same procedure as in our previous study (15), and to test the relationship between sensitivity to sensory perturbations and balance. The results may be useful in the design of appropriate sensory rehabilitation programmes after stroke.
METHODS
Participants
The patient group comprised 30 subjects (21 men, 9 women), mean age 54.7 years (standard deviation (SD) 10.6) (range 26–74 years). All patients were hospitalized in our physical and rehabilitation medicine unit. They had recently (less than 6 months previously) experienced their first and only cerebral hemispheric stroke, with various sequelae including motor and balance impairments. They were included as soon as they could stand without assistance or assistive devices, for 3 trials of 70 s. Patients were not included if they were over 75 years of age, had reduced alertness or a pre-stroke history of neurological disturbance, vertigo, vestibular dysfunction, amblyopia or diplopia. Each patient was informed of the procedure, but aphasic patients were excluded before the inclusion if they could not understand the procedure. Before testing, subjects were asked to provide consent. A complete neurological examination was then carried out including: motor impairment, using the Motricity Index (18), functional independence using the Barthel Index (BI) (19) and visual field, assessed at the bedside and confirmed by Goldman campimetry when a visual field defect was suspected. The joint position sense for the ankle and the knee was evaluated. Patients were then classified into 2 groups in relation to sensitivity: normal sensitivity for position joint sense (group S1) and abnormal sensitivity, even when impairment was slight (group S2). Balance and functional mobility, were evaluated, initially and 1 month later, using the Berg Balance Scale (BBS) (20) and the Timed Up and Go (TUG) test (21). A computed tomography (CT) scan or magnetic resonance imaging (MRI) of the brain and brain stem was carried out for each patient, and if any tumours, or pathologies of the posterior fossa, were found, these subjects were excluded. Thirty patients were included (Table I): 13 had a right hemispheric lesion (RHL) and 17 a left hemispheric lesion (LHL). The CT scan or MRI showed that the stroke was haemorrhagic in 13 patients and ischaemic in 17. All of the ischaemic lesions involved the middle cerebral artery.
The control group comprised 30 healthy subjects (14 men, 16 women), mean age 52.0 years (SD 12.0). They had no musculoskeletal, vestibular, visual or somatosensory impairments. None of the control subjects was taking medication known to interfere with alertness or postural control. Twenty of them had participated in the previous study. They were age-matched with the stroke patients. Twelve control subjects were re-tested 4–6 weeks later.
The study was reviewed and approved by the Comité de Protection des Personnes, Ile de France IV (number 2007/28). The subjects signed an informed consent form before participating.
Clinical Trial Registration: NCT01146249 (this study is a sub-study of the full study registered).
Quantification of postural responses to sensory perturbations
Balance was evaluated using a double force platform (FeeTest from Techno Concept, Mane, France) consisting of 2 adjacent force platforms on which subjects stood barefoot in double-leg stance with their feet placed parallel, 12 cm apart, each on 2 force transducers that recorded the vertical ground reaction forces. The placement of the feet was drawn on the ground to ensure a constant placement during all trials. Subjects were asked to stand at ease and to look straight ahead with their head erect and their arms hanging by their sides. The position of the centre of pressure (CoP) was calculated from the ground reaction force. Data were collected with a sampling frequency of 40 Hz.
The hemiparetic subjects were tested as soon as possible after the onset (session 1) of stroke and were re-tested after 4–6 weeks during a rehabilitation programme (session 2), which included repetitive and high intensity static and dynamic exercises with multisensory perturbations focused on balance and gait training in our Physical and Rehabilitation Medicine (PRM) department (22).
The evaluation lasted approximately 1 h and was divided into 3 parts involving proprioceptive, visual and vestibular perturbations. The 3 types of perturbations challenged postural control both in the pitch (antero-posterior) and roll (medio-lateral) planes; 12 perturbations (4 proprioceptive, 4 visual, and 4 vestibular perturbations) were successively tested in the same order for all subjects so as to induce postural sway to the right, to the left, forwards and backwards (Table I). Each trial began with a 15-s baseline period, with no perturbation, followed by a 35-s period of perturbation, and a final 20-s period of observation with no perturbation.
Proprioceptive perturbation (Table I)
Electromagnetic Vibrators (VB 115, Techno Concept) were positioned on the tendon of the muscles to be stimulated. Each cylindrical vibrator head was 7 cm long and 3 cm in diameter. Mechanical vibrations (pulse duration: 5 ms, amplitude: 1 mm peak to peak) were delivered at a frequency of 50 Hz on the triceps surae and tibialis anterior and at 90 Hz on the gluteus medius. These frequencies were chosen after a pilot investigation had demonstrated that it induces a sufficient displacement to be registered. Vibratory perturbation was applied in a bright room, eyes open, on the tendons of the 2 triceps sural, 2 tibialis anterior and the right and then left gluteus medius muscles.
|
Table I. Experimental design. Three types of sensory perturbations challenging postural control were tested so as to induce sway to the right, to the left, forwards and backwards |
||||
|
Sensory perturbations |
Type of perturbations |
Sessions |
Site of stimulation |
Direction of the displacement |
|
Proprioceptive |
Vibration |
1 |
Triceps |
Backwards |
|
2 |
Anterior tibialis |
Forwards |
||
|
3 |
Left gluteus medius |
Rightward |
||
|
4 |
Right gluteus medius |
Leftward |
||
|
Visual |
Optokinetic |
5 |
Top to bottom |
Forwards |
|
6 |
Bottom to top |
Backwards |
||
|
7 |
Clockwise |
Rightward |
||
|
8 |
Counterclockwise |
Leftward |
||
|
Vestibular |
Galvanic |
9 |
Cathode on right |
Leftward |
|
10 |
Cathode on left |
Rightward |
||
|
11 |
Cathode on right, turned to the right |
Forwards |
||
|
12 |
Cathode on right, head turned to the left |
Backwards |
||
Visual perturbation (Table I)
Optokinetic perturbation (OKS) was performed in a dark room with no visual reference cues. OKS was induced using numerous luminous spots, produced by a rotating sphere (Optotest, Techno Concept) placed just above the patient’s head and projected on the wall in front of them. The speed of rotation was 60°/s. Subjects were instructed to stare straight ahead at the stimulus pattern without attempting to follow the moving dots. Four types of OKS were tested; the movement of the luminous dots was first oriented linearly from top to bottom, from bottom to top, and, finally, circularly clockwise then counterclockwise.
Vestibular perturbation (Table I)
Binaural bipolar galvanic vestibular perturbation (GVS) was delivered to the subjects by 9-cm2 rectangular Ag-AgCl pre-gelled disposable electrodes placed over each mastoid in a bright room, with the eyes open. The electrodes were secured with adhesive tape and an elastic bandage wrapped around the head. The GVSs were trapezoidal: 3-s ascending ramp; 29-s plateau; 3-s descending ramp. Four types of galvanic perturbation inducing a displacement in the 4 directions were tested: 2 perturbations in the frontal plane with the head facing forwards, with the cathode placed first on the right mastoid process, then on the left mastoid process; and 2 perturbations in the sagittal plane with the head turned to the right and then to the left, with the cathode always placed on the side of the cerebral lesion (or on the right side for the control subjects).
Force platform data
The characteristics of the displacement of the projection of the CoP were analysed during the proprioceptive, visual and vestibular perturbations in the first and second sessions.
The displacement (in mm) of the CoP during the perturbation was calculated as the mean position of the CoP during the perturbation (from 15 to 50 s) minus the mean position of the CoP in the initial resting period (from 2 to 13 s). The mean displacement was successively calculated for each direction (anterior, posterior, right and left) and for each type of sensory perturbation (visual, proprioceptive and vestibular) and expressed as an absolute value. A multidirectional score (in mm) was then calculated for each type of sensory perturbation as the mean of the absolute value of the displacement recorded in the anterior, posterior, right and left directions during the first and second sessions. The sensory scores for proprioceptive, visual and vestibular perturbations were termed Proprio Score1, Visual Score1 and Vestib Score1 for the first session, and termed Proprio Score2, Visual Score2 and Vestib Score2 for the second session. A global sensory score 1 and a global sensory score 2 were also calculated as the sum of the 3 multidirectional sensory scores. Higher scores indicate a greater sensitivity to the sensory perturbations, i.e. greater perturbation of balance during application of the stimulus. The 75th percentile was arbitrarily chosen to define a threshold of sensitivity to a sensory stimulation. When a sensory score was greater than the 75th percentile of the sensory scores found in the control subjects, the subject was considered to be sensitive to that type of stimulation.
Statistical analysis
The reproducibility of the sensory scores was first assessed in 12 control subjects using the intra-class correlation coefficient (ICC). Reproducibility is considered to be good if the ICC is above 0.6 and excellent if above 0.8.
The sensory scores were summarized by their means and standard deviations (SD). Patients’ scores were first compared with the scores of the matched healthy subjects using a Wilcoxon test. A Mann-Whitney test was then used to compare the groups according to gender and side of lesion. Spearman’s correlation was used to investigate if there was any relationship between sensory scores, age, and time since stroke, sensation, motor control, and postural scales. Sensory scores obtained initially and 1 month later were then compared using a Wilcoxon test. The level of significance was fixed at 5% and SAS StatView software© was used for all the analyses.
Results
Control subjects
The value of the ICC for the visual and the vestibular multidirectional sensory scores during the perturbation was considered to be excellent: 0.93 for the Visual score and 0.80 for the Vestib score. However, the reproducibility of the Proprio score was low because the perturbation of the gluteus medius was difficult to obtain in some subjects. Therefore, the results for proprioceptive perturbation in the lateral plane were excluded and a good level of reproducibility was then obtained with an ICC multidirectional score of 0.71. As expected, a large degree of inter-individual variability was found in control subjects in response to sensory perturbations.
Patients’ characteristics
Motor and balance control were fairly good for the majority of patients. Proprioceptive sensation was normal in 16 patients and impaired in 14. Patients with RHL and LHL were comparable with regard to age, sex, time since stroke, motor control, and sensation (Table II).
|
Table II. Characteristics of the patients |
|||
|
Total patients Mean (SD) |
LHL (n = 17) Mean (SD) |
RHL (n = 13) Mean (SD) |
|
|
Age, years |
54.7 (10.6) |
53.0 (12.13) |
55.8 (8.5) |
|
Time since stroke, months |
1.9 (1.2) |
1.6 (1.2) |
2.2 (1.1) |
|
Motricity index (maximum 100) |
65.3 (19.9) |
65.4 (20.3) |
65.2 (20.1) |
|
BBS1 (maximum 56) |
46.2 (7.0) |
46.7 (6.9) |
45.6 (7.5) |
|
TUG1, s |
28.7 (22.6) |
25.0 (10.9) |
33.3 (31.7) |
|
Barthel Index1 (maximum 100) |
82.6 (17.3) |
80.2 (19.8) |
85.7 (13.6) |
|
BBS: Berg Balance Scale; TUG Timed-up and Go test; LHL: patients with left hemispheric lesion; RHL: patients with right hemispheric lesion. Age, time since stroke, Motricity, BBS, TUG and Barthel Index were summarized by their median and interquartile range distance. |
|||
Initial sensitivity to sensory perturbations of the hemiparetic patients
The multidirectional scores for stroke subjects are shown in Fig 1. This figure shows the subjects ranked according to increasing sensitivity to the perturbations, using the global sensory score 1 as an index.
The hemiparetic patients were globally more sensitive to the different sensory perturbations than the control subjects (mean global score1 = 48.4 (SD 18.9) vs 34.8 (SD 15.8), p = 0.001. The Proprio Score1 was not significantly different between the hemiparetic patients and control subjects (mean Proprio Score1 = 18.1 (7.9) vs 18.4 (12.0), p = 0.749); the Visual Score1 was significantly higher in hemiparetic patients than in control subjects (mean Visual Score1 = 21.1 (13.1) vs 9.7 (7.1), p = 0.0008); The Vestib Score1 was not significantly different between hemiparetic patients and control subjects (mean Vestib Score1 = 10.1 (8.8) vs 6.5 (5.3), p = 0.082). However, it is important to stress that, as for control subjects, the patients’ postural reactions to the proprioceptive, visual and vestibular perturbations were heterogeneous, with sensory scores (Proprio Score1, Visual Score1, and Vestib Score1) varying greatly between patients (Fig. 1).
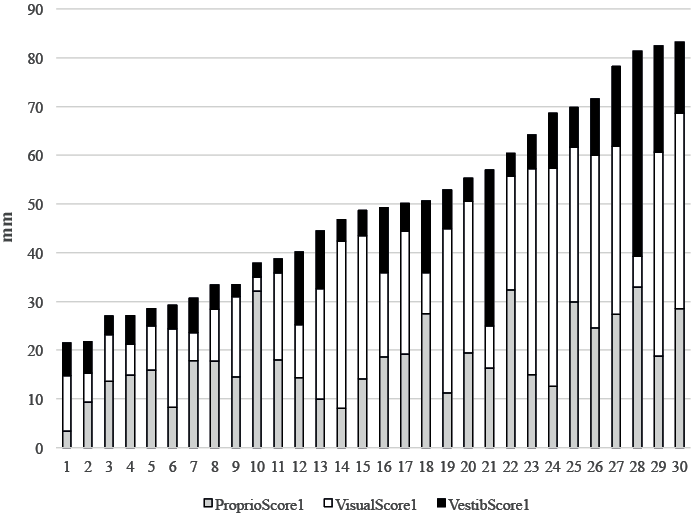
Fig. 1. Inter-individual variability of responses to sensory perturbations in each stroke patient. Initial multidirectional scores (in mm) of each subject during the proprioceptive (grey), visual (white) and vestibular (black) perturbations recorded by the platform (PF). Stroke subjects were ranked according to increasing sensitivity to the proprioceptive (vibratory), visual (optokinetic perturbation) and vestibular perturbations (galvanic perturbation), using the global sensory score as an index.
Changes in sensitivity to sensory perturbations of the hemiparetic subjects
The sensory evaluations were repeated 4–6 weeks after the first test for 26 hemiparetic subjects. Four patients dropped out; 3 because of the occurrence of a medical complication (recurrence of stroke, melanoma, and bone marrow aplasia) and 1 was lost to follow-up.
The mean global sensory score decreased significantly between the first and second evaluations (p = 0.001), although the reduction in sensory perturbation was not significant (Fig. 2).
One month later, the mean global score was no longer different from these of the control subjects (p = 0.77). The difference between the patients and the control subjects for Proprio Score2 and Vestib Score 2 was not significant (respectively, p = 0.602 and p = 0.151). The difference between the patients and the control subjects for Visual Score2 remained significant (p = 0.033).
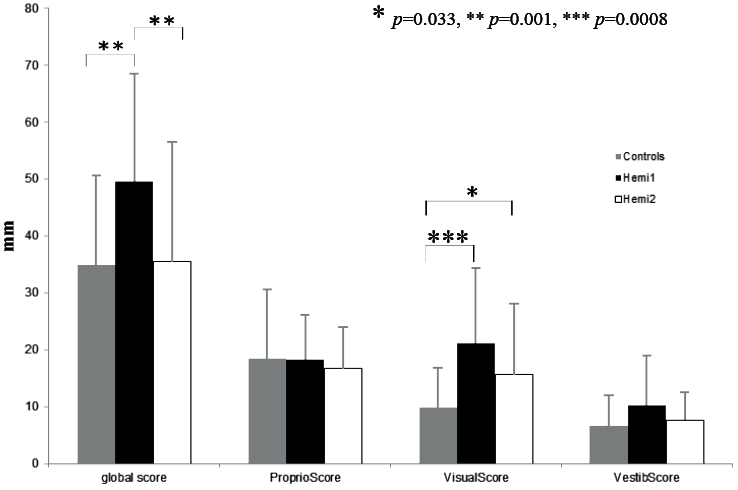
Fig. 2. Changes in the sensory scores of the patients with stroke. Values (mean, standard deviation) of the sensory scores for control subjects (grey), for patients with stroke at the first evaluation (black) and for patients with stroke at the second evaluation (white).
The percentage of patients (27%) sensitive to proprioceptive stimulation was the same as initially, 47% of patients were sensitive to visual stimulation (vs 60% initially) and 35% of patients were sensitive to vestibular stimulation (vs 43% initially).
Influence of the patients’ characteristics
No correlations were found between the patients’ characteristics (age, sex, time since stroke, side of lesion, motor impairment, sensation) and the sensory scores, except between the Motricity Index and the Proprio Score1 (Rs = 0.47, p = 0.01) and between time since stroke and the Proprio Score1 (Rs = –0.47, p = 0.01).
Relationship between sensitivity to sensory perturbations and balance
Proprio Score1 was correlated with the TUG1 (Rs = –0.412, p = 0.029) and Vestib Score1 was correlated with the BBS1 (Rs = –0.368, p = 0.047) and the Barthel 1 (Rs = –0.513, p = 0.005). There were no significant correlations between Visual Score1 and BBS1 (Rs = –0.344, p = 0.063) or the Barthel 1 (Rs = –0.336, p = 0.070).
One month later, Proprio Score2 was no longer correlated with any balance tests. Vestib Score2 was correlated with BBS2 (Rs = –0.576, p = 0.006), TUG2 (Rs = 0.408, p = 0.045), Barthel2 (Rs = –0.481, p = 0.016) and Visual Score2 was significantly correlated with BBS2 (Rs = –0.500, p = 0.019), and with TUG2 (Rs = 0.401, p = 0.049), but not with the Barthel2 (Rs = –0.355, p = 0.075).
At the second session, the mean Motricity Index score was correlated with the balance tests, but not with any of the sensory scores. However, when stroke patients were separated in 2 equal subgroups of motor ability (Motricity Index score above or equal to the median value (68 points), there was a strong relationship between Visual Score2 and BBS2 (Rs = –0.72, p = 0.01) and between Vestib Score2 and BBS2 (Rs = –0.6, p = 0.04) in the group with poor motor ability. These relationships were not significant in the group with good motor ability (Rs = –0.3, p = 0.3 and Rs = 0.4, p = 0.1, respectively). That is, the relationship between sensory score and balance was stronger in the more impaired hemiparetic patients.
DISCUSSION
In a recent study, we showed that, after a recent stroke, the postural reactions of the patients to visual, vestibular and proprioceptive perturbations were initially more pronounced than for the control subjects (15). The aim of this study was to investigate the stability of this perceptual behaviour. In order to do so, the standing posture of 30 patients after a first hemispheric stroke was challenged, with the same experimental design as in our previous study using proprioceptive, visual and vestibular perturbations, as soon as they could stand without assistance or assistive devices, then one month later. Reproducibility of the perturbations was tested in 12 control subjects and was considered as good to excellent once the gluteus medius tendon perturbations were excluded.
Sensibility to sensory perturbations after stroke
The results of the present study confirmed those of our previous study, showing that once they have acquired independent standing ability, stroke patients express an excessive sensitivity to sensory perturbations regarding balance control. We also found that hypersensibility to one sensory perturbation does not necessarily entail neglect of the 2 other sensory information, and that patients use individual perceptual behaviour with respect to balance control. Visual information was particularly greatly used, initially (approximately 60% of patients) and also 1 month later (approximately 50% of patients), by most of the patients with stroke. This is in line with previous studies in which an abnormal reliance on visual input was found, even late after stroke (8, 13, 14). During the first months after stroke, vestibular hypersensitivity was also frequent (40% of patients). This heightened sensitivity to galvanic vestibular stimulations fits with the results of the study of Marsden et al. (23): the lateral forces generated by galvanic vestibular stimulation in the stroke group were enhanced on the side of the non-paretic limb and diminished on the side of the paretic limb, which was not the case with controls. Finally, the sensibility to proprioceptive perturbation is comparatively less pronounced. This is in line with the results of Di Fabio, who found that proprioceptive information could be used as efficiently as in a control group to improve postural control in stroke patients deprived of visual information (24). We also found that, the higher the motor and balance level, the more sensitive the patients are to proprioceptive stimulation. In addition, the more sensitive the patients are to proprioceptive stimulation, the shorter is the delay in acquiring the capacity to stand independently. Therefore, perceptual behaviour consisting in high sensitivity to proprioceptive perturbation could be appropriate and could demonstrate an adequate perceptual motor control. On the other hand, patients could develop excessive sensitivity to visual and vestibular perturbations because the lesion could cause perturbations of the corporal scheme built on an egocentric frame of reference (25), which could lead to increased use of external feedback rather than centrally driven feed-forward mechanisms.
Recovery of sensitivity to sensory perturbations
One month later, sensitivity to sensory perturbations had greatly decreased. Most patients had probably returned to their pre-stroke sensory profile. The sensitivity to vestibular and proprioceptive perturbations are close to normal values, the only sensory score which is different from control subjects is the visual score. Visual information continues to be used excessively by a lot of the patients with stroke. Perhaps because this external information requires a less complex integration process and is more independent from motor control (24). Indeed, visual information is often used, even in healthy subjects, in new or challenging balance tasks, and in children at each new postural acquisition (26–29).
Relationship between sensitivity to sensory perturbations and balance
During the first months after stroke, both visual and vestibular sensibility are associated with poor balance. This relationship was found to be stronger one month later. We also found that the relationship between sensory score and balance was stronger in the more impaired hemiparetic patients. Although these results should be interpreted with caution because of the high number of scores calculated, they raise the issue of causality between increased visual or vestibular hypersensitivity and balance. One could argue that increased visual or vestibular hypersensitivity could induce poor postural control because it exposes the patients to be in difficulties in keeping their balance in case of sensory conflict or deprivation of external sensory information. Conversely, patients could progressively learn to re-weight sensory information and increase the use of external feedback rather than centrally driven feed-forward mechanisms because of poor balance. This learned behaviour could be considered maladaptative if the patients became too reliant on one source of information and underused other preserved sensory capacities. On the other hand, it could be an adapted strategy for the patients to cope with their new sensorimotor deficits and limitation in activities, notably for the most impaired patients (15).
Consequences for balance rehabilitation of patients with stroke
Recent post-stroke patients expressed excessive sensitivity to sensory perturbations during balance maintenance. However, perceptual behaviour was not stable for most patients during the first post-stroke months. Therefore the initial months following stroke appear to be a period of individual perceptual motor adaptation and sensory re-weighting is likely to be a major component of that process. These results suggest that balance rehabilitation after stroke should include sensory rehabilitation programmes early after stroke designed to promote perceptual motor adaptation and prevent maladaptive sensory strategies from developing. Rehabilitation programmes could be focused on intensive multisensory programmes to challenge perceptual-motor control. Such programmes have been carried out using repetitive and high-intensity static and dynamic exercises focused on balance and gait under various sensory input manipulations (visual deprivation, foam rubber track, unstable platforms, cervical movements) (22, 30). Rehabilitation programmes should also be individually designed in order to enhance the integration of underused elementary sensory input and to reduce the overuse of other afferences (31). Some studies have suggested that a rehabilitation programme employing visual deprivation so as to promote the use of somatosensory and vestibular inputs may reduce visual reliance and improve function (31, 32). The use of sensory conflict conditions is also likely to be beneficial (33, 34). Finally, because the lesion could cause perturbations of the corporal scheme built on an egocentric frame of reference, which could cause increased use of external feedback rather than centrally driven feed-forward mechanisms, the use of feed-forward, rather than feedback mechanisms could be stimulated for balance control by reinforcing the use of spatial references (25, 30).
Conclusion
The results confirmed that recent post-stroke patients expressed excessive sensitivity to sensory perturbations during balance maintenance. The development of visual and vestibular dependence is frequent after stroke and could contribute to poor balance. However, perceptual behaviour was not stable for most patients during the first-post stroke months. Therefore the initial months following the stroke appear to be a period of individual perceptual motor adaptation, and sensory re-weighting is likely to be a major component of that process. Rehabilitation programmes should then be designed to improve the postural reactions to sensory perturbations and prevent maladaptive sensory strategies from developing.
The authors declare no conflicts of interest.
REFERENCES
