Willem M. Star1, Albert J. van ‘t Veen2, Dominic J. Robinson1, Kai Munte2, Ellen R. M. de Haas2 and Henricus J. C. M. Sterenborg1
1Photodynamic Therapy and Optical Spectroscopy Program, Department of Radiation Oncology, Daniel den Hoed Oncology Centre, and 2Department of Dermatology, Erasmus MC, Rotterdam, The Netherlands
Photodynamic therapy (PDT) of superficial basal cell carcinoma using topical 5-aminolaevulinic acid (ALA) and 75–100 J/cm2 light dose yields unsatisfactory long-term results. In several animal models, illumination with two light fractions approximately 2 h apart was considerably more effective than single illumination, suggesting the need for a pilot clinical study. Fifteen patients with a total of 86 primary superficial basal cell carcinomas, received topical ALA and were illuminated 4 and 6 h later, both with 45 J/cm2 laser light (633±1 nm). Fluorescence spectra were measured before and immediately after each illumination. At a mean follow-up of 59 months (range 44–82), 67 lesions could be evaluated, 56 of which showed a complete response (84%). Cosmesis was good/excellent in 88% of the complete response group and fair in 12%. There was no correlation between protoporphyrin fluorescence and response, but a significant correlation between the percentage of fluorescence left after photobleaching by the first illumination and the amount of protoporphyrin re-synthesized 2 h later. In conclusion, the long-term complete remission rate of fractionated ALA-mediated PDT of superficial basal cell carcinoma as reported here is significantly better than after PDT with single illumination previously reported by others, but equal to studies using single illumination with a much higher light fluence. Further improvement may be possible by reducing the fluence of the first fraction, with constant total fluence. Key words: 5-aminolaevulinic acid; fluorescence; fractionated illumination; photodynamic therapy, basal cell carcinoma.
(Accepted April 18, 2006.)
Acta Derm Venereol 2006; 86: 412–417.
Dominic J. Robinson, PhD, Center for Optical Diagnostics and Therapy Department of Radiation Oncology, Erasmus MC, Room wk 319, PO Box 2040, NL-3000 CA, Rotterdam, The Netherlands. E-mail: d.robinson@erasmusmc.nl
Photodynamic therapy (PDT) of superficial non-malignant and malignant skin lesions, using topically applied 5-aminolaevulinic acid (ALA) to endogenously generate the photosensitizer protoporphyrin IX (PpIX), was introduced by Kennedy et al. in 1990 (1). Since then, this subject has received increasing attention. Initially, high (80–100%) complete response (CR) rates were reported for topical ALA-mediated photodynamic therapy (ALA-PDT) in the treatment of superficial non-melanoma skin cancer (1–5). To achieve high CR rates, lesions were sometimes treated more than once (2, 3, 6, 7). Follow-up periods were short (approximately 3–12 months). Long-term CR rates were less satisfactory. Fink-Puches et al. (8) reported a recurrence rate of 44% (36/81) after a median follow-up of 19 months (range 3–60) of superficial basal cell carcinoma (sBCC) treated by ALA-PDT. The success of topical ALA-PDT depends on several factors. In addition to penetration of ALA into the skin (lesion) and the formation of a therapeutic concentration of PpIX, CR depends significantly on tumour thickness and duration of ALA application (9). Curettage prior to topical ALA-PDT may significantly improve CR rates, in particular for nodular basal cell carcinoma (nBCC) (10, 11). Oseroff (12) emphasized the importance of light fluence in the treatment of sBCC by topical ALA-PDT. He reported that a CR rate of 95% after 200 J/cm2 (at a fluence rate of 150 mW/cm2) fell to 70% after 150 J/cm2. Most other investigators applied lower light fluences.
In recent years derivatives of ALA have been studied as pro-drugs for PDT. One of these pro-drugs, ALA-methylester, has also been studied clinically (13). When ALA-methylester enters the cell, it is first converted to ALA and subsequently to PpIX. The photosensitizer used is therefore the same as with ALA-PDT, but since the pharmacokinetics and localization of ALA-methylester is different from ALA, the clinical outcome regarding selectivity and efficacy of PDT may also be different.
Unsatisfactory clinical results led Cairnduff et al. (14) and Haller et al. (15) to introduce routine double topical ALA-PDT treatments with an interval of one week, yielding considerably increased CR rates. The CR rate of 79% (median follow-up 13 months) after topical ALA-PDT of sBCC reported from our group (4) did not last (unpublished data) so we have also looked at ways to improve the treatment. A therapeutic light fluence completely bleaches the PpIX-fluorescence. During clinical treatments of patients with multiple lesions we noticed, however, that a few hours after the illumination, some lesions showed fluorescence again, suggesting re-synthesis of PpIX and the opportunity to perform a second illumination. This was subsequently confirmed in animal studies (16–18). The re-synthesis of PpIX suggested the application of a second illumination, a few hours after the first, in the hope of increasing the effectiveness of ALA-PDT. Increased efficacy of ALA-PDT by fractionated illumination with intervals between 75 min and 6 h was indeed demonstrated in several animal models: first, using systemically administered ALA, on tumour and normal rat skin in the window chamber model (16) and on subcutaneous tumours in the flank of rats (19); secondly, using topically applied ALA on normal and UVB-treated hairless mouse skin (18, 20, 21); and thirdly, using locally injected ALA on normal pigskin (22). In the present paper we show clinical results for the treatment of sBCC using topically applied ALA and two light fractions with a 2-h interval.
MATERIALS AND METHODS
Patients
The ethical review committee of the Erasmus University Medical Center approved the study, which was conducted according to the Declaration of Helsinki. Informed consent was obtained from each patient. Fifteen patients, all Caucasian, with a total of 86 biopsy proven (3 or 4 mm punch) primary sBCC were included. The lesions, which had not been treated before, were located on the trunk (28), scalp (27), leg (18), face (11) and arm (2). There were 8 men and 7 women with a mean age of 61 years at the time of the first fractionated ALA-PDT treatment session (range 35–83 years). Two patients had 1 lesion, 3 patients had 2 lesions, 2 patients had 9 lesions and the remaining 8 patients had 3, 4, 5, 6, 7, 8, 10 and 17 lesions, respectively. Each lesion was photographed before ALA application and at each follow-up visit. A transparent plastic sheet was used to mark the lesion and pigmented spots or other useful markers in the vicinity, for easy identification at follow-up visits. For patient 6 a transparent plastic helmet was made on which the lesions were marked. Lesion sizes were determined clinically. From the smallest (a) and the largest (b) dimension, using the ellipse formula, the area was calculated, i.e. area = πab/4. An equivalent lesion diameter was calculated using the circle formula, i.e. equivalent circle diameter = √(4×area/π). The dimensions of the lesions ranged from 4 to 50 mm. The mean equivalent diameter was 13 mm.
5-aminolaevulinic acid application and local anaesthetic
ALA solution (20% w/w) was prepared by the hospital pharmacy, dissolving ALA hydrochloride (Sigma, Zwijndrecht, The Netherlands) in Instillagel® (Medeco BV, Oud Beijerland, the Netherlands). Instillagel® was used because it contains lidocaine, which might alleviate pain during the therapeutic illumination and it also has a convenient viscosity for topical application. The pH of the ALA solution was not adjusted (pH ≈ 2.1). Thin gauze was cut to cover the lesion with at least 5 mm margin of clinically normal skin and was soaked in the ALA solution. After painting ALA solution onto the lesion and the margin, the area was covered with the gauze, which was fixed in place and occluded with Tegaderm (3M Healthcare, Loughborough, UK). If a surface crust was present prior to treatment the patient was asked to apply salicylic acid 10% ointment the night before the treatment to facilitate bloodless removal of the crust before ALA application. The Tegaderm® was covered with aluminium foil and a bandage to prevent uncontrolled effects of ambient light on PpIX.
The possibility of pain during illumination was explained to each patient. Then the patient was given the choice of no medication, a lidocaine injection or topical application of EMLA (Astra Zeneca, Zoetermeer, The Netherlands), a cream containing lidocaine and prilocaine. In the latter case, 45–60 min before the illumination the gauze with ALA was removed and the remaining ALA solution wiped off with a wad of cotton wool. A thin layer of EMLA® was applied and covered with Tegaderm®. This was removed just before illumination. If pain relief by EMLA® was insufficient, local lidocaine injection was applied to lesions treated subsequently. Analgesic measures were only needed for the first illumination, the second usually being less painful. Of 86 lesions treated, 38 were pre-treated with EMLA®, 26 with lidocaine injection and 22 treatments were performed without analgesic medication.
Illumination
The light source was a Spectra Physics 365B dye laser (Spectra Physics, Darmstadt, Germany) pumped by a 2040E Argon ion laser. The dye laser was tuned to a wavelength of 633±1 nm, which is the local maximum of the fluorescence excitation spectrum of PpIX (4). We have found that the wavelength of the maximum in the action spectrum for PDT using haematoporphyrin derivative (HPD) is the same as the maximum in the fluorescence excitation spectrum of HPD (i.e. 625 nm) (23, 24). Since the fluorescence excitation spectrum can be determined more easily and more precisely than the action spectrum, we measured the former for PpIX and assumed that the wavelength of the maximum would also represent the maximum of the PDT-action spectrum, as for HPD. The difference between 633±1 nm and the optimum wavelength of 635±2 nm as reported by Szeimies et al. (25) is not clinically significant.
The laser light was coupled into a 600 µm optical fibre and subsequently projected onto the lesion using a lens so as to ensure a uniform fluence rate across the beam diameter. The lesion and a margin of at least 5 mm around it were illuminated at a constant fluence rate of 50 mW/cm2 to a fluence of 45 J/cm2 for both light fractions. The protocol prescribed an interval of 4–6 h between the ALA application and the first illumination (including EMLA application). This was realized (mean 5.0 h) with one exception (6.5 h). The second illumination was to be given 2 h after the first. Practical constraints resulted in 1.5–2.5 h intervals with a vast majority of 2 h (mean 2.0 h).
Fluorescence measurements
Fluorescence spectra of BCC were measured before ALA application, immediately before and after the first illumination and immediately before and after the second illumination. The treatment room was darkened to avoid interference of ambient light with the fluorescence measurements. For technical reasons, spectra could only be obtained from 48 of the 86 treated lesions. Excitation light (405±5 nm) was obtained from a filtered xenon arc lamp and was coupled into a bifurcated fibre optic (no. 77558, Oriel, Stratford, CT, USA). The central fibre was used for fluorescence excitation and a surrounding ring of fibres was used for detection. The fibre end was mounted on an aluminium cylinder with a length of approximately 30 mm, an outer diameter of 40 mm and with a 14 mm diameter, 24.5 mm deep hole in the centre to accommodate the hand-piece of the fibre optic. The hole continued with a diameter of 10 mm through the remaining 5.5 mm of the cylinder. Thus, the hand-piece with excitation and detection fibres could reproducibly be placed on a lesion to be measured and a 2.8 mm diameter excitation light spot could reproducibly be projected onto the lesion. On the bifurcated side of the fibre optic the ring of detection fibres is converted to a line, which is imaged on the slit of a monochromator. The spectra were recorded with an Instaspec IV monochromator plus CCD detector system (Oriel). The fluence rate of the excitation light was 2 mW/cm2 and the measurement took one second per spectrum so that the fluorescence measurements did not cause any detectable photobleaching or photodynamic effects. Measurements on a sheet of white plastic were used as a reference to correct the fluorescence measurements of BCC for any changes in the output of the excitation light source or the sensitivity of the detection system.
The autofluorescence spectrum taken before each ALA application was subtracted from the PpIX fluorescence spectra taken before and after each illumination. The PpIX fluorescence spectra were then analysed as a linear combination of basis fluorescence spectra, as described by Robinson et al. (18).
Follow-up
According to the protocol, treatment results were evaluated clinically at 1 week, 1 month, 3, 6, 9, 12, 18, 24, 36, 48 and 60 months. Photographs were taken at each follow-up visit, unless there was no change. A biopsy was taken only if a lesion was suspected of residual or recurrent BCC. In the latter case the lesion would not be retreated by PDT, but by surgery. The cosmetic result was scored as good/excellent (+), fair (0) or poor (–). Score “+” was assigned when the healed lesion could not be distinguished from the surrounding skin. In a few cases (legs) the cosmetic aspect of normal skin was not very good, but since the healed skin at the treated BCC site could not be distinguished from the surrounding normal skin, a “+” score was nevertheless assigned. Scarring would be scored poor, but did not occur. The only adverse cosmetic effect seen was hypo-pigmentation, which was scored fair.
RESULTS
Response to fractionated ALA-PDT
The first analysis of the fractionated clinical ALA-PDT data was performed when the minimum follow-up was one year and the final analysis approximately 2.5 years later.
Seventy-six out of 86 lesions (88%) showed a complete clinical response with a median follow-up of 32 months (mean 30, range 12–51 months). Patient 3 died intercurrently of a cause unrelated to BCC. The follow-up data included above (8/8 CR) refer to the last time this patient was seen by the dermatologist, approximately one year before the first analysis.
Two patients were not available for the final analysis. These were patients 3 (above) and 6 (8/9 CR), whose helmet (see the Patients section) was accidentally destroyed and whose scalp was surgically treated elsewhere. Furthermore, patient 9 (4/6 CR) was referred for plastic surgery shortly after the first analysis because of multiple new non-superficial BCCs on the scalp. Two sBCC sites with CR were included in the surgery (full scalp skin transplant). These were therefore excluded from the final follow-up data set. Thus, the final analysis included 13 patients with 67 sBCCs, 56 CR (84%) and 11 recurrences. The median follow-up was 58 months (mean 59, range 44–82 months). The present result is significantly better than that reported by Fink-Puches et al. (8), i.e. 45/81 or 56% (p = 0.0003, two-tailed, Fisher’s exact test). See the Discussion for further comments.
Recurrences were observed 12 months (2 cases), 13, 17 (2), 18, 21, 32 (2), 48 and 61 months after PDT. The total number of recurrences was 12. The analysis at a minimum follow-up of 12 months included 10 recurrences. Two more occurred after that. For the final analysis the data of two patients had to be excluded (see above) including one recurrence, so that there were 11 recurrences in the final analysis. For three recurrences the interval between ALA application and first illumination was less than 5 h. For all treated lesions this number was 27 (out of 86). The fraction of lesions with a short first interval is therefore not larger for the recurrences than for the complete responses. The interval between first and second illumination was 2 h for all recurrences except one (2.25 h). A relationship between recurrences and different intervals, as reported in the literature (9) therefore cannot be demonstrated in the present study.
Normally redness and some crust formation following ALA-PDT treatment heal within 2–3 weeks. One lesion of patient 13 occurred in an area that had received radiotherapy for bladder cancer, 25 years before PDT. The reaction (blisters, haemorrhage) was much stronger than normal and the wound became larger than the treated area. Healing occurred after application of Fucidine (fusidic acid) ointment, and was complete about 2 months after PDT. Eventually, there was no sign of scarring and the skin was hardly distinguishable from normal. At the first analysis the cosmetic score was good/excellent in 67 of CR cases (88%) and fair in 9 cases (12%). No lesions showed a poor cosmetic result. In 7 out of 9 cases a "fair" score was assigned because of (slight) hypo-pigmentation, 4 of which were pre-existing, i.e. caused by the BCC. There was no change in the cosmetic score of CR-lesions at the final follow-up. One of the recurrences in the final analysis occurred at a site that had previously received cosmetic score "+" (patient 12), the other had received score "0" (patient 7).
Fluorescence
The PpIX fluorescence just before the first light fraction varied considerably, between the lesions of each patient as well as between patients. In arbitrary units, the mean fluorescence is 2.00 (SD 1.30, range 0.25–5.01). There is no apparent relationship between the magnitude of the measured lesion fluorescence and the clinical response (CR or recurrence). The recurrences fall into two groups. (A) One lesion that showed hardly any photobleaching during the first illumination, that subsequently synthesized relatively more PpIX during the dark interval and showed the most intense fluorescence of any lesion before the second light fraction (see Fig. 1). PpIX fluorescence in this lesion was completely bleached during the second illumination. (B) The other lesions that showed much more photobleaching during the first illumination and little re-synthesis in the dark interval.
When the fluorescence of all lesions before the first illumination is normalized to 100%, the mean fluorescence after the illumination is 16% (SD 18, range 0–81). The mean fluorescence before the second illumination is then 54% (SD 59, range 2–291) and the fluorescence after the second illumination is 5% (SD 7, range 0–39). There is a rather wide variation in photobleaching during the first light fraction despite fixed illumination parameters. Just like the fluorescence before the first illumination, the fluorescence before the second illumination varies considerably, even after normalization as presented above.
In a previous animal study (18) it was found that if the fluence of the first light fraction was reduced (i.e. less photobleaching), re-synthesis of PpIX in the dark interval increased. In the present study the light fluence of the first and second illuminations were constant, but because of the large variations in fluorescence, it was possible to test the data of the present study for a similar effect. There is a significant correlation between the percentage of PpIX fluorescence photobleached by the first light fraction and the normalized fluorescence of re-synthesized PpIX after a 2-h dark interval, just before the second light fraction (Fig. 1, p ≤ 0.0008, Spearman rank correlation). That is, when the percentage of fluorescence photobleached by the first light fraction is reduced, the normalized fluorescence just before the second light fraction increases and this trend is significant. This confirms clinically, what Robinson et al. (18) measured experimentally on hairless mouse skin.
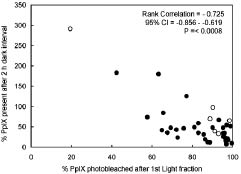
Fig. 1. Fluorescence present after the 2-h dark interval, expressed as percentage of protoporphyrin IX (PpIX) fluorescence before the first illumination, plotted against the percentage of PpIX fluorescence photobleached after the first light fraction, showing a significant correlation. Open symbols: recurrences, closed symbols: complete responses.
DISCUSSION
To dissolve ALA, a range of different water containing solvents has been used clinically, such as oil in water emulsions (1, 5, 7) and cream bases (3, 26). We used Instillagel®, a water containing gel, as solvent. There is no evidence that different solvents lead to differences in efficacy of PDT, except possibly the addition of dimethylsulphoxide, which appears to improve ALA penetration into the skin and the remission rate of thin nBCCs (10). If the solvent affects the clinical efficacy of ALA-PDT, comparison between studies will be more difficult.
The cosmetic outcome was of particular concern in this study, because fractionated topical ALA-PDT could cause scarring in hairless mouse skin (20). No scarring was, however, observed in the present study. Hairless mouse skin is probably more susceptible to scarring because it is much thinner than human skin.
The short-term CR rate of the present study (minimum follow-up of 12 months) is 76/86 or 88%. This is not significantly better than our previous result (4) using a single illumination, i.e. 33/42 or 79% (p = 0.19, two-tailed, Fisher’s exact test), nor is it significantly different from that of Haller et al. (15), for example, who applied double treatments with a one week interval and obtained a short-term CR rate of 25/26 or 96% (p = 0.45).
More important is our long-term CR rate of 56/67 or 84%. This is significantly better than the long-term response rate reported by Fink-Puches et al. (8), i.e. 45/81 or 56% (p = 0.0003). There are no other reports on long-term response of sBCC to topical ALA-PDT with which our results can be statistically compared. The total applied light fluence in our study was only 90 J/cm2. This is much less than the fluence recommended (> 150 J/cm2) by Oseroff et al. (12, 26) who achieved high long-term CR rates using non-fractionated ALA-PDT. Our method has an advantage here, because application of a large light fluence in a reasonable time requires a very powerful (laser) light source, in particular for large lesions, and such a light source is not always available.
The double treatment protocol of Haller et al. (15) has also been used with favourable results in PDT of nBCC with topical ALA-methylester as pro-drug (13). Double treatments take double time, require two drug applications and are therefore more costly and less convenient for patients. Our light fractionation protocol, using a single ALA-application, takes a little less than one day and only slightly more time than a single illumination.
Babilas et al. (27) found no increased effectiveness of fractionated PDT with an interval of 15 minutes and intravenous (i.v.) ALA, whereas in a similar model we observed increased PDT-damage using an interval of 90 min between fractions (16). The 15-min interval (27) is probably too short. Curnow et al. (28), illuminating rat colon with a flat-cut optical fibre, observed more extended necrosis upon fractionated ALA-PDT with a 150 second interval (i.v. ALA), whereas Robinson et al. (18, 29) have seen no significant difference between non-fractionated and fractionated ALA-PDT of hairless mouse skin with a 2-min interval (topical ALA). Given this apparent contradiction, we calculated that in the rat colon model (28), using point source illumination, the total fractionated light fluence at the boundary of the largest area of necrosis may be up to a factor of 10 less than the single fluence at the boundary of the smallest area of necrosis. This situation is entirely different from the skin and other flat geometries (16, 18, 27), where the fluence in the tissue layers of interest does not depend very much on depth. Considering the consistency of our present and previous results (16, 18), light fractionation effects with a 150-second interval may be a particularity of the animal model used (28).
Fluorescence could be measured only on 48 of the 86 treated lesions, because of availability of equipment. Given the large variations in the results it is unlikely that the interpretation of the data would have been different if all lesions could have been measured. The correlation presented in Fig. 1 confirms experimental data (18) and occurred despite the large variations in fluorescence. Had more lesions been measured, the significance would most likely have improved.
The large variations in fluorescence may have been caused by several factors, such as variations in tissue optical properties, in oxygenation and heterogeneity of the PpIX distribution in the lesions (30). Such variability is widely observed in ALA-induced fluorescence of normal human skin and skin lesions (30–33).
Model calculations suggest (34) that the fluorescence after the 2-h dark interval can be as large as the fluorescence before the first illumination, that is 100% in terms of Fig. 1. Since PDT causes cell damage, the PpIX generating capacity of these cells is probably reduced and less fluorescence is expected after the 2-h dark interval. Figure 1 shows, however, that this fluorescence can be 200% or more. Clearly there is an effect, probably cellular, that causes a large increase in the availability of ALA and/or its conversion into PpIX as well as an increase in the effectiveness of the second illumination. The mechanisms are not necessarily the same, because the largest fluorescence after the 2-h dark interval is associated with a recurrence (Fig. 1). We also note that our superficial fluorescence measurements using 405 nm excitation do not report effects that may occur at the base of the lesion, particularly in thicker lesions, where local effects may have a critical relationship to recurrence.
Our studies on light fractionation were prompted by re-appearance of fluorescence some time after illumination and concomitant photobleaching in clinical ALA-PDT of BCC, just as reported by Orenstein et al. (33). Initially we thought that the increased amount of PpIX available for the treatment could explain the increased biological effect of light fractionation, but this fails because of the absence of correlation between fluorescence and response. As yet a satisfactory explanation is lacking. According to Curnow & Bown (35) reperfusion injury is involved in the mechanism of ALA-PDT with single as well as fractionated illumination. A similar study with our models might elucidate possible analogies and differences between the colon model and other models in this respect. Although vascular effects in ALA-PDT are not as pronounced as in PDT with haematoporphyrin derivatives and other photosensitizers, vasoconstriction has been reported after single illumination (16, 20) and haemorrhage after fractionated illumination (16), so that reperfusion injury may also be a factor here.
In summary, despite all the remaining questions, fractionated topical ALA-mediated PDT, as presented in this study, appears to be a step towards effective treatment of sBCC. Further improvements may be possible by reducing the percentage of the total fluence that is delivered in the first fraction (21).
ACKNOWLEDGEMENTS
The authors wish to thank Riëtte de Bruijn and Angélique van der Ploeg for their help with the fluorescence measurements. D. J. Robinson’s contribution to this work was funded by the Dutch Cancer Society, projects no. DDHK 98-1686 and EMCR 02-2718.
Conflicts of interest: None reported.
REFERENCES
1. Kennedy JC, Pottier R, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B: Biol 1990; 6: 143–148.
2. Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B: Biol 1992; 14: 275–292.
3. Svanberg K, Andersson T, Killander D, Wang I, Stenram U, Andersson-Engels S, et al. Photodynamic therapy of nonmelanoma tumours of the skin using topical δ-aminolaevulinic acid sensitisation and laser irradiation. Br J Dermatol 1994; 130: 743–751.
4. Meijnders PJN, Star WM, De Bruijn HS, Treurniet-Donker AD, Van Mierlo MJM, Wijthoff SJM, et al. Clinical results of photodynamic therapy for superficial skin malignancies and actinic keratosis using topical 5-aminolaevulinic acid. Lasers Med Sci 1996; 11: 123–131.
5. Wennberg A-M, Lindholm LE, Alpsten M, Larkö O. Treatment of superficial basal cell carcinomas using topically applied delta-aminolevulinic acid and a filtered xenon lamp. Arch Dermatol Res 1996; 288: 561–564.
6. Calzavara-Pinton PG. Repetetive photodynamic therapy with topical delta-aminolaevulinic acid as an appropriate approach to the routine treatment of superficial non-melanoma skin tumours. J Photochem Photobiol B:Biol 1995; 29: 53–57.
7. Morton CA, Whitehurst C, Moseley H, McColl JH, Moore JV, Mackie RM. Comparison of photodynamic therapy with cryotherapy in the treatment of Bowen's disease. Br J Dermatol 1996; 135: 766–771.
8. Fink-Puches R, Soyer HP, Hofer A, Kerl H, Wolf P. Long term follow-up and histological changes of superficial nonmelanoma skin cancers treated with topical δ-aminolevulinic acid photodynamic therapy. Arch Dermatol 1998; 134: 821–826.
9. Morton CA, MacKie RM, Whitehurst C, Moore JV, McColl JH. Photodynamic therapy for basal cell carcinoma: effect of tumor thickness and duration of photosensitizer application on response. Arch Dermatol 1998; 134: 248–249.
10. Soler AM, Warloe T, Tausjø J, Berner A. Photodynamic therapy by topical aminolevulinic acid, dimethylsulphoxide and curettage in nodular basal cell carcinoma: a one year follow-up study. Acta Derm Venerol 1999; 79: 204–206.
11. Thissen MRTM, Schroeter CA, Neumann HAM. Photodynamic therapy with delta-aminolaevulinic acid for nodular basal cell carcinoma using a prior debulking technique. Br J Dermatol 2000; 142: 338–339.
12. Oseroff AR. PDT for cutaneous malignancies: clinical applications and basic mechanisms. Photochem Photobiol 1998; 67: 17–18 (abstract SAM-D8).
13. Rhodes LE, de Rie M, Enström Y, Groves R, Morken T, Goulden V, et al. Photodynamic therapy using topical methyl aminolevulate vs surgery for nodular basal cell carcinoma. Br J Dermatol 2004; 140: 17–23.
14. Cairnduff F, Stringer MR, Hudson EJ, Ash DV, Brown SB. Superficial photodynamic therapy with topical 5-aminolaevulinic acid for superficial primary and secondary skin cancer. Br J Cancer 1994; 69: 605–608.
15. Haller JC, Cairnduff F, Slack G, Schofield J, Whitehurst C, Tunstall R. Routine double treatments of superficial basal cell carcinomas using aminolaevulinic acid-based photodynamic therapy. Br J Dermatol 2000; 143: 1270–1274.
16. Van der Veen N, van Leengoed HLLM, Star WM. In vivo fluorescence kinetics and photodynamic therapy using 5-aminolaevulinic acid-induced porphyrin: increased damage after multiple irradiations. Br J Cancer 1994; 70: 867–872.
17. Van der Veen N, de Bruijn HS, Star WM. Photobleaching during and re-appearance after photodynamic therapy of topical ALA-induced fluorescence in UVB-treated mouse skin. Int J Cancer 1997; 72: 110–118.
18. Robinson DJ, de Bruijn HS, de Wolf WJ, Sterenborg HJCM, Star WM. Topical 5-aminolevulinic acid photodynamic therapy of hairless mouse skin using two-fold illumination schemes: PpIX fluorescence kinetics, photobleaching and biological effect. Photochem Photobiol 2000; 72: 794–802.
19. De Bruijn HS, van der Veen N, Robinson DJ, Star WM. Improvement of systemic 5-aminolevulinic acid-based photodynamic therapy in vivo using light fractionation with a 75-minute interval. Cancer Res 1999; 59: 901–904.
20. Van der Veen N, Hebeda KM, de Bruijn HS, Star WM. Photodynamic effectiveness and vasoconstriction in hairless mouse skin after topical 5-aminolevulinic acid and single or two-fold illumination. Photochem Photobiol 1999; 70: 921–929.
21. Robinson DJ, de Bruijn HS, Star WM, Sterenborg HJCM. Dose and timing of the first light fraction in two-fold illumination schemes for topical ALA-mediated photodynamic therapy of hairless mouse skin. Photochem Photobiol 2003; 77: 319–323.
22. Thissen MR, de Blois MW, Robinson DJ, de Bruijn HS, Dutrieux RP, Star WM, et al. PpIX fluorescence kinetics and increased skin damage after intracutaneous injection of 5-aminolevulinic acid and repeated illumination. J Invest Dermatol 2002; 118: 239–245.
23. Star WM, Versteeg AAC, van Putten WLJ, Marijnissen JPA. Wavelength dependence of hematoporphyrin derivative photodynamic treatment effects on rat ears. Photochem Photobiol 990; 52: 547–554.
24. Star WM. In vivo action spectra, absorption and fluorescence excitation spectra of photosensitizers for photodynamic therapy. J Photochem Photobiol B: Biol 1995; 28: 101–102.
25. Szeimies R-M, Abels C, Fritsch C, Karrer S, Steinbach P, Bäumler W, et al. Wavelength dependency of photodynamic effects after sensitization with 5-aminolevulinic acid in vitro and in vivo. J Invest Dermatol 1995; 105: 672–677.
26. Oseroff AR, Shieh S, Frawley NP, Cheney R, Blumenson LE, Pivnick EK, et al. Treatment of diffuse basal cell carcinomas and basaloid follicular hamartomas in nevoid basal cell carcinoma syndrome by wide area 5-aminolevulinic acid photodynamic therapy. Arch Dermatol 2005; 141: 60–67.
27. Babilas P, Schacht V, Liebsch G, Wolfbeis OS, Landthaler M, Szeimies, R-M, et al. Effects of light fractionation and different fluence rates on photodynamic therapy with 5-aminolaevulinic acid in vivo. Br J Cancer 2003; 88: 1462–1469.
28. Curnow A, McIllroy BW, Postle-Hacon MJ, MacRobert AJ, Bown SG. Light dose fractionation to enhance photodynamic therapy using 5-aminolevulinic acid in the normal rat colon. Photochem Photobiol 1999; 69: 71–76
29. Robinson DJ, de Bruijn HS, van der Veen N, Stringer MR, Brown SB, Star WM. Protoporphyrin IX fluorescence photobleaching during ALA-mediated photodynamic therapy of UVB-induced tumors in hairless mouse skin. Photochem Photobiol 1999; 69: 61–70.
30. Martin A, Tope WD, Grevelink JM, Starr JC, Fewkes JL, Flotte TJ, et al. Lack of selectivity of protoporphyrin IX fluorescence for basal cell carcinoma after topical application of 5-aminolevulinic acid: implications for photodynamic treatment. Arch Dermatol Res 1995; 287: 665–674.
31. af Klinteberg C, Enejder AMK, Wang I, Andersson-Engels S, Svanberg S, Svanberg K. Kinetic fluorescence studies of 5-aminolevulinic acid-induced protoporphyrin IX accumulation in basal cell carcinomas. J Photochem Photobiol B: Biol 1999; 49: 120–128.
32. Golub AL, Gudgin Dickson EF, Kennedy JC, Marcus SL, Park Y, Pottier RH. The monitoring of ALA-induced protoporphyrin IX accumulation and clearance in patients with skin lesions by in vivo surface-detected fluorescence spectroscopy. Lasers Med Sci 1999; 14: 112–122.
33. Orenstein A, Kostenich G, Malik Z. The kinetics of fluorescence during ALA-PDT in human malignant skin tumors. Cancer Lett 1997; 120: 229–234.
34. Star WM, Aalders MC, Sac H, Sterenborg HJCM. Quantitative model calculation of the time dependent protoporphyrin IX concentration in normal human epidermis after delivery of ALA by passive topical application or iontophoresis. Photochem Photobiol 2002; 75: 424–432.
35. Curnow A, Bown SG. The role of reperfusion injury in photodynamic therapy with 5-aminolaevulinic acid – a study on normal rat colon. Br J Cancer 2002; 86: 989–992.
