Claus Dam1 and Anette Bygum2
Departments of 1Rheumatology and 2Dermatology, Odense University Hospital, Sdr. Boulevard 29, DK-5000 Odense C, Denmark. E-mail: Claus.Dam@ouh.regionsyddanmark.dk
Accepted June 4, 2007.
Claus Dam1 and Anette Bygum2
Departments of 1Rheumatology and 2Dermatology, Odense University Hospital, Sdr. Boulevard 29, DK-5000 Odense C, Denmark. E-mail: Claus.Dam@ouh.regionsyddanmark.dk
Accepted June 4, 2007.
Sir,
Proton pump inhibitors (PPIs), especially omeprazole, lansoprazole and pantoprazole, are important agents used for eradicating Helicobacter pylori, treating peptic ulcer and gastroesophageal reflux disease. They are generally well tolerated, but side-effects occur in up to 5% of patients, mainly as headache, diarrhoea and nausea (1). Fewer than 15 articles reporting cutaneous side-effects have been published, including 2 cases of cutaneous lupus erythematosus (CLE) (2, 3). We report here 5 additional cases of subacute cutaneous lupus erythematosus (SCLE) induced or exacerbated by PPIs, with a detailed summary of 2 cases.
CASE REPORTS
Case 1
A 63-year-old woman with Addison’s disease had been substituted with per-oral hydrocortisone, 25 mg daily, for 41 years. Fourteen years earlier she had an attack of SCLE, and since then sunlight could provoke CLE. In May 1998 pantoprazole, 40 mg daily, was initiated because of oesophagitis, and after 3 days she developed an annular rash on her chest, which spread to the whole trunk, neck and face after 4 weeks (Fig. 1). Screening for antinuclear antibodies (ANA) and histone antibodies was negative, but she had positive anti-Ro/SSA antibodies (SSA) (38 UI/ml; normal <2 UI/ml). Two years earlier a screening for ANA had been positive, with a speckled pattern not further specified. Histological examination of a skin biopsy demonstrated a lymphocytic interface dermatitis, with vacuolar degeneration of basal keratinocytes, keratinocyte necrosis and a perivascular infiltrate of lymphocytes in the superficial dermis, diagnostic of CLE. Direct immunofluorescence (DIF) was negative. Topical steroids, increased dose of per-oral hydrocortisone (50 mg daily) and hydroxychloroquine, 250 mg daily, were prescribed. Pantoprazole was stopped and the rash cleared completely within 4 weeks. The patient recalled that a few years earlier she had been treated with pantoprazole, and at that time had developed a similar rash, which cleared shortly after the treatment was stopped. The previous rash had not been examined by a dermatologist.
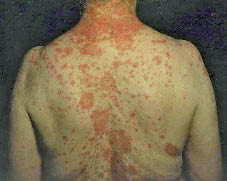
Fig. 1. Papulosquamous and annular subacute cutaneous lupus erythematosus in patient 1.
Case 2
A 57-year-old man had a 22-year tendency to recurrent sun-induced rash on his upper chest and face, sometimes associated with myalgias and arthralgias. He had been treated with felodipine, warfarin and citalopram for 5 years without influence on this tendency to skin rash. In August 1997 an endoscopy showed a duodenal ulcer and therefore lansoprazole, 30 mg daily, was started. Four weeks later he developed a widespread papulosquamous reddish-blue confluent exanthema, distributed on the trunk and upper and lower extremities (Fig. 2) without accompanying systemic symptoms. Laboratory tests showed positive ANA with a speckled pattern, positive SSA (40 UI/ml), and marginal positive anti-DNA antibodies (5.9 mg/l; normal <5 mg/l). Screening for anti-histone antibodies was negative. Skin biopsy showed CLE and DIF presented IgG and C3 deposits along the dermo-epidermal junction. At that time lansoprazole was not suspected as a cause, and treatment was continued. Despite treatment with hydroxychloroquine, 250–500 mg daily, potent topical steroids and per-oral prednisolone 30 mg daily, the rash did not clear. Any attempt to reduce the dosage of per-oral prednisolone caused a flare of the exanthema, and azathioprine 100 mg daily was added. The patient was followed-up by a dermatologist in private practice. The patient died more than 2 years later, with persistent active skin disease and ongoing lansoprazole treatment.
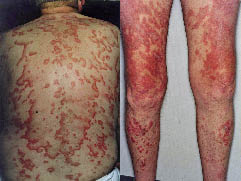
Fig. 2. Widespread circinate and gyrate papulosquamous exanthema in patient 2.
Other cases
In a 4-year period from September 1997 to July 2001 we saw further 3 patients with SCLE probably induced by PPIs. A summary of the clinical and para-clinical data in all 5 patients is shown in Table I.
Table I. Clinical, serological and histological characteristics in five cases of subacute cutaneous lupus erythemathosus (SCLE) induced or exacerbated by proton pump inhibitors
|
Case no. Sex/age (years) |
Lupus criteria |
Culprit drug |
Latencya |
Relevant serology |
Biopsy |
Course |
||
|
Previous |
In relation to rash |
After recovery |
||||||
|
1 F/63 |
Photosensitivity ANA+ |
Pantoprazole |
3 days |
ANA+ speckled |
ANA– SSA+ SSB– Histone– |
ANA– SSA+ (after 16 months) |
CLE, DIF– |
CR in 4 weeks |
|
2 M/57 |
Photosensitivity ANA+ dsDNA+ |
Lansoprazole |
4 weeks |
n.d. |
ANA+, speckled SSA+, dsDNA+ histone-, RF+, LA+ |
ANA+ homogeneous (after 9 months) |
CLE, DIF+ |
Active SCLE up to death year 2000 |
|
3 F/61 |
DLE ANA+ |
Lansoprazole |
3 weeks |
ANA+ speckled dsDNA– |
ANA+, speckled SSA+, SSB–, dsDNA– histone–, RF+ |
ANA– (after 13 months) |
CLE, DIF+ |
CR in 12 weeks |
|
4 F/50 |
Malar rash, arthritis, pleuritis, proteinuria, seizures, psychosis ANA+, dsDNA+ (Diagnosed with SLE) |
Omeprazole |
7 weeks |
ANA+ homogeneous dsDNA+ |
ANA+ homogeneous dsDNA+, histone– |
ANA+ homogeneous (after 13 months) |
Not performed |
CR in 4 weeks |
|
5 F/51 |
Photosensitivity ANA+ |
Pantoprazole |
4–8 weeks |
ANA+ speckled dsDNA– |
ANA+, speckled SSA–, SSB–, dsDNA– RF+ |
n.d. |
Erythema multiforme-like CLE, DIF– |
Active SCLE up to death year 2001 |
aLatency between culprit drug introduction and cutaneous lupus erythematosus (CLE) onset; + positive; – negative.
CR: complete clinical recovery; DLE: discoid lupus erythematosus; SLE: systemic lupus erythematosus; ANA: antinuclear antibodies; SSA: Anti-Ro/SSA antibodies; SSB: Anti-La/ SSB antibodies; dsDNA: antibodies to double-stranded DNA; histone: anti-histone antibodies; RF: rheumatoid factor; LA: lupus anticoagulans; DIF: direct immunofluorescence; n.d.: no data.
DISCUSSION
Lupus erythematosus (LE) is a chronic inflammatory autoimmune disease with a wide spectrum of manifestations, including CLE. SCLE is a subtype of CLE that usually manifests as annular, polycyclic erythematous scaly plaques or confluent papulosquamous (psoriasiform) lesions with limited systemic involvement (4, 5). SCLE is typically associated with positive ANA, SSA, anti-La/SSB antibodies (SSB) findings and correlates with certain HLA types (A1, B8, DR3) (5). A lesional skin biopsy is usually characterized by a lymphocytic interface dermatitis with vacuolar degeneration of the epidermal basal layer and necrotic keratinocytes (5, 6). DIF eventually plays a minor role, due to the low specificity and sensitivity and to the expanding spectrum of serological testing (7). DIF and serological tests may be supportive, but neither is diagnostic. The diagnosis is based upon clinical and histopathological correlation.
The first case of drug-induced SCLE was recognized in 1985 after hydrochlorothiazide treatment (8), and subsequently an increasing number of drug-induced SCLE cases has been reported after treatment with thiazides, ACE inhibitors, calcium channel blockers, terbinafine, statins and other drugs (9, 10). Two cases of lansoprazole-induced SCLE have been published recently in this journal (3). In contrast to drug-induced systemic LE, anti-histone antibodies are of minor importance in drug-induced SCLE (5).
We have presented here 2 cases, and a summary of 3 other cases, with SCLE possibly related to PPIs. These cases were found in relation to a retrospective medical chart review. One patient had a pre-existent history of SLE, while the other patients probably had a predisposition to LE, e.g. arthralgias, ANA+, photosensitivity or CLE. A characteristic clinical picture of SCLE was found in 4 of these 5 patients. Patient 5 presented clinical and histological signs consistent with erythema multiforme-like SCLE, which could be a variant of Rowell’s syndrome (11), or an additional morphological SCLE-form, as suggested by Massone et al. (12). Patient 4 had underlying SLE, but developed drug-induced SCLE. A drug correlation is supported by the fact that skin rashes in patients with SLE are mostly of the acute CLE type. A lesional skin biopsy confirmed the diagnosis of SCLE in patients 1–3 and 5, but was not performed in patient 4. All except patient 1 had positive ANA screening, with a speckled pattern in patients 2, 3 and 5, and a homogeneous pattern in patient 4. Patients 1–3 had positive SSA. Measurement of SSA/SSB was not performed in patient 4. Anti-histone antibodies were measured in 4 of the 5 patients and were negative. ANA-measurements previous to skin rash had been performed in 4 patients, showing positive ANA with a speckled pattern in patients 1, 3 and 5, and a homogeneous pattern in patient 4. Earlier SSA measurements were not performed.
When suspecting drug-induced SCLE, coincidental idiopathic SCLE and other drug rashes should be borne in mind. Idiopathic and drug-induced SCLE are difficult to differentiate, but Bonsmann et al. (13) suggested that involvement of the lower extremities is suspicious for drug-induced SCLE. All of our patients had unusually widespread and inflamed elements, making a drug correlation suggestive. Most important for diagnosing drug-related SCLE is the historical and temporal connection between symptoms and the suspected drug. The delay between introduction of the suspected drug and onset of CLE in our cases varied between 3 days and 7 weeks. The short latency in patient 1 can be explained by re-exposure. The skin rash in patients 1, 3 and 4 cleared completely within 4–12 weeks after withdrawal of the PPI. This time course is in accordance with previous observations in drug-induced SCLE (3, 9, 10). The possibility of PPIs and other drugs as causative factors has not always been central in the mind of doctors at our institution; hence in patients 2 and 5 the PPIs were not discontinued. Both patients died of other causes, but with active SCLE, after treatment with PPIs for up to 2 years. Some patients received several drugs, but we think that a culprit drug could be determined as other medication was not altered during the observation period. Besides withdrawal of the suspected drug, the patients were concomitantly treated with potent topical steroids, anti-malarial drugs (hydroxychloroquine) and per-oral prednisolone in variable doses according to common guidelines (14).
Other environmental factors can induce SCLE (4, 5). As far as we know, none of the patients altered their behaviour towards possible triggers, such as ultraviolet radiation and cigarette smoking, during the observation period. We routinely informed patients about sun protection measures.
The onset or exacerbation of SCLE in these 5 patients is probably due to PPIs, because of the close temporal relationship between introduction of the drug and onset of symptoms, clearance of the cutaneous eruption shortly after stopping PPIs in 3 of 5 patients and no clearance of the rash in the 2 patients in whom PPIs were continued. In earlier articles a possible photosensitizing potential of PPIs has been mentioned, and this could be one of the mechanisms triggering CLE (2, 15).
The cases described here suggest that clinicians should be aware of this rare side-effect of PPI, especially in patients with a history of LE or predisposing host factors, since recognition and discontinuation of the suspected drug, together with relevant treatment of the CLE will result in clearance of the widespread lupus rash, while continuation of PPI treatment will cause a persistent skin rash with a high requirement for immunomodulating topical or systemic treatment.
ACKNOWLEDGEMENT
The authors thank Dr Ole Clemmensen, Department of Pathology, Odense University Hospital, for his expertise in the histological analysis of skin biopsies.
REFERENCES
1. Reilly JP. Safety profile of the proton-pump inhibitors. Am J Health-Syst Pharm 1999; 56 Suppl 4: S11–17.
2. Raison-Peyron N, Minh HB, Peyron JL, Demoly P, Guillot B. Toxidermie photodistribuée induite par les inhibiteurs de la pompe à protons. Thérapie 2005; 60: 85–87.
3. Bracke A, Nijsten T, Vandermaesen J, Meuleman L, Lambert J. Lansoprazole-induced subacute cutaneous lupus erythematosus: two cases. Acta Derm Venereol 2005; 85: 353–354.
4. Kuhn A, Gensch K, Stander S, Bonsmann G. Cutaneous lupus erythematosus. Part 1: clinical manifestations and classification. Hautarzt 2006; 57: 251–268.
5. Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev 2005; 4: 253–263.
6. Crowson AN, Magro C. The cutaneous pathology of lupus erythematosus: a review. J Cutan Pathol 2001; 28: 1–23.
7. Parodi A, Caproni M, Cardinali C, Bernacchi E, Fuligni A, De Panfilis G, et al. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. A review by the Italian group of immunodermatology. Dermatology 2000; 200: 6–10.
8. Reed BR, Huff JC, Jones SK, Orton PW, Lee LA, Norris DA. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med 1985; 103: 49–51.
9. Srivastava M, Rencic A, Diglio G, Santana H, Bonitz P, Watson R, et al. Drug-induced Ro/SSA-positive cutaneous lupus erythematosus. Arch Dermatol 2003; 139: 45–49.
10. Antonov D, Kazandjieva J, Etugov D, Gospodinov D, Tsankov N. Drug-induced lupus erythematosus. Clin Dermatol 2004; 22: 157–166.
11. Rowell NR, Beck JS, Anderson JR. Lupus Erythematosus and erythema multiforme-like lesions. A syndrome with characteristic immunological abnormalities. Arch Dermatol 1963; 88: 176–180.
12. Massone C, Parodi A, Rebora A. Erythema multiforme-like subacute cutaneous lupus erythematosus: a new variety? Acta Derm Venereol 2000; 80: 308–309.
13. Bonsmann G, Schiller M, Luger TA, Stander S. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol 2001; 44: 925–931.
14. Callen JP. Cutaneous lupus erythematosus: a personal approach to management. Australas J Dermatol 2006; 47: 13–27.
15. Cockayne SE, Glet RJ, Gawkrodger DJ, McDonagh AJ. Severe erythrodermic reactions to the proton pump inhibitors omeprazole and lansoprazole. Br J Dermatol 1999; 141: 173–175.
