Psoriasis is an inflammatory skin disease in which dysregulation of p63, a member of the p53 family that is crucial for skin development and maintenance, has been demonstrated. Involvement of miR-203, miR-21 and miR-125b, small non-coding RNAs implicated in the regulation of p63 or p53, has been suggested in the pathogenesis of psoriasis. To elucidate the roles of p63 and p63-related microRNAs in psoriasis and to increase our understanding of the mechanisms of narrow-band ultraviolet B (NB-UVB) phototherapy, we studied the effects of NB-UVB treatment on the expression of these molecules. Skin biopsies from 12 psoriasis patients were collected before, during and after NB-UVB therapy. Real-time PCR and immunohistochemistry showed that p63 expression was not significantly affected, whereas NB-UVB phototherapy significantly decreased expression of miR-21 (p = 0.003) and increased miR-125b levels (p = 0.003). The results indicate that the unresolved p63 abnormality in treated epidermis may play a role in maintenance of this disease. Key words: psoriasis; NB-UVB phototherapy; p63; microRNA.
(Accepted December 1, 2010.)
Acta Derm Venereol 2011; 91: XX–XX.
Karin Nylander, Department of Medical Biosciences/Patho- logy, Umeå University, Bldg 6M, 2nd floor, SE-901 85 Umeå, Sweden. E-mail: karin.nylander@medbio.umu.se
Psoriasis is a common disease affecting approximately 2% of the population worldwide (1). It is a chronic inflammatory and hyperproliferative skin disorder that is immunomediated and genetically determined (2). Though temporarily fully reversible with appropriate therapy, psoriasis can last for decades with altered interactions between resident skin cells and elements of the immune system (3). Current treatments for psoriasis targeting epidermal keratinocytes and/or mononuclear leukocytes, two fundamental cell types involved in psoriasis pathogenesis, are effective in most patients (3, 4). Narrow-band ultraviolet B (NB-UVB) phototherapy is commonly used in the treatment of psoriasis, and induction of T-cell apoptosis has been shown as the main mechanism by which NB-UVB resolves lesions (5–7). NB-UVB irradiation could also cause apoptosis in cultured epidermal keratinocytes (8). However, the mechanisms underlying these processes have not been completely elucidated.
The response of various cells to UV radiation is a highly complex issue. Many studies have focused on p53 transcription factor, because of its pivotal role in triggering cell cycle arrest or inducing apoptosis in cells damaged by UV irradiation. In psoriasis lesions, elevated p53 accumulation has been reported (9, 10), and following NB-UVB phototherapy, an increase in p53 expressing keratinocytes has been seen (11). Similar to p53, p63 also transcriptionally regulates specific genes that mediate cell cycle arrest and apoptosis upon DNA damage, cooperating with or independent of p53 (12). p63 is a member of the p53 transcription factor family, and is expressed as six different isoforms, including three variants containing the N-terminal transcription-activation domain (TAp63α, TAp63β and TAp63γ) and three variants lacking this domain (∆Np63α, ∆Np63β and ∆Np63γ) (13). p63 plays an essential role in development and maintenance of skin and other epithelial tissue (14–17). Distinct functions of p63 variants are crucial for epidermal homeostasis by mediating cell adhesion and regulating proliferation, differentiation and cell survival (18, 19). Notably, after UVB irradiation, levels of ∆Np63α are decreased in keratinocytes (20, 21), whereas TAp63 levels increase due to UVC irradiation (22, 23). The effect of clinical NB-UVB phototherapy on p63 expression has, however, not been studied.
MicroRNAs (miRNA) are a large family of small non-coding RNAs which can negatively regulate gene expression by inhibiting translation of the mRNA targets or promoting mRNA degradation (24). The contribution to psoriasis of miRNAs, such as miR-21, miR-125b, miR-203, miR-221 and miR-222, has been suggested previously (25, 26). Interestingly, several miRNAs have been shown to inhibit expression of p63 or p53, which is critical in the regulation of cell cycle arrest and/or apoptosis. For example, miR-203 can promote keratinocyte differentiation or control cancer cell survival upon genotoxic damage by repressing the expression of ∆Np63 (27, 28). Down-regulation of miR-21 in glioblastoma cells led to repression of growth, increased apoptosis and cell cycle arrest, by regulation of TAp63 expression, as seen at both mRNA and protein levels (29). miR-125b can negatively regulate p53 protein levels, thus regulating p53-induced apoptosis during development and stress response (30).
We have previously shown p63 to be dysregulated in psoriasis (31). In order to further elucidate its role in this disease and to increase our understanding of the mechanisms involved in NB-UVB therapy of psoriasis, we examined p63 levels in psoriatic epidermis following phototherapy. With the aim of mapping the importance of miRNAs in treatment of psoriasis, we further examined levels of p63 or p53 regulating and psoriasis-related miRNAs.
MATERIALS and METHODS
NB-UVB phototherapy
Twelve patients diagnosed with plaque-type psoriasis were recruited to the study. Individual minimum erythema doses (MEDs) were assessed before the treatment. NB-UVB irradiation was administered to the whole body two to three times a week using a cabinet (PCL 8000, Puva Combi Light – ARKADE, Heverlee, Belgium) equipped with fluorescent lamps (UVB TL100W/01, Philips, Eindhoven, The Netherlands). Treatment comprising approximately 24 sessions was given during a period of 2–3 months, with a starting dose of 0.1–0.3 J/cm2, increased subsequently depending on skin tolerance and clinical response. Clinical improvement was assessed by evaluating erythema, desquamation and induration after phototherapy. The patients’ clinical data are summarized in Table SI (available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1086).
Skin samples
A total of six punch biopsies with a diameter of 4 mm were taken from lesions on each psoriasis patient: two prior to UV treatment (pre-UV), two at the middle of UV treatment (mid-UV) and two before the last session of UV treatment (post-UV). Even if lesions in some patients had almost resolved post-UV, affected skin could be distinguished from normal skin. One patient left at the middle stage of phototherapy, thus only four biopsies were collected. Twelve healthy age- and sex-matched volunteers were also recruited and two punch biopsies taken from the buttocks. Samples were divided and immediately embedded in Tissue Tek OCT compound (Miles Inc., Elkhart, Indiana, USA), snap-frozen in liquid nitrogen and stored at –80ºC. The study was approved by the ethics committee of Umeå University (UmU Dnr 08–108 M) and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all subjects.
Microdissection
Samples were cut into 10 µm thick sections and placed on membrane-covered glass slides (PALM Membrane Slides, PALM Microlaser Technologies, Bernried, Germany). Slides were then placed in 75% ethanol, followed by diethylpyrocarbonate (DEPC)-treated water and stained with HistoGene staining solution (Arcturus Bioscience, Mountain View, CA, USA). After a short rinse in water and dehydration in ethanol, slides were air-dried and stored dry. All reagents were kept on ice. Microdissection was carried out using a PALM® microlaser system (PALM GmbH, Bernried, Germany). Epidermis or the basal layers of epidermis (2–3 cell layers including the basal cell layer) were isolated from each specimen and placed in separate microtubes containing Trizol reagent (Invitrogen, Stockholm, Sweden) and stored at –80ºC.
Total RNA extraction and cDNA synthesis
Total RNA was extracted following the Trizol protocol, and the MinElute Spin Column supplied in the Qiagen Rneasy micro kit (Qiagen, GmbH, Hilden, Germany) was used for RNA clean-up. The modified RNA clean-up protocol was used to collect total RNA containing small RNAs. Synthesis of cDNA was performed using 200 ng total RNA with First-Strand cDNA Synthesis Kit (USB, Cleveland, USA). For miRNA analysis, 10 ng of template RNA diluted to a final concentration of 2 ng/μl was reverse transcribed using First-strand cDNA synthesis kit and miR-specific RT primers from the miRCURY LNATM microRNA polymerase chain reaction (PCR) system (Exiqon, Vedbaek, Denmark) according to the manufacturer’s instructions.
Quantitative reverse transcription polymerase chain reaction
The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 3’:5’ assay was used to estimate mRNA integrity (32). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using an IQ5 multicolour RT-PCR detection system with IQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA). Sequences of primers used for GAPDH quantification are from Nolan et al. (32). Primers for p53 and p63 are shown in Table SII (available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1086). Up-regulation of fatty acid binding protein 5 (FABP5) has previously been shown in psoriasis (33) and accordingly used as positive control when comparing psoriasis and control samples. Each sample was measured in duplicate and the results were normalized in relation to the geometric mean of three reference genes RPL13A, TUBA6 and GAPDH. Mercury LNATM microRNA assays (Exiqon), which is sensitive and accurate in detection of miRNA by quantitative PCR using SYBR Green, were used to quantify miR-203, miR-125b and miR-21, respectively, using U48 as endogenous control. All RT-PCR data were quantified by calculating fold change using the ∆∆CT method.
Immunohistochemical staining
A monoclonal antibody directed against p63 (4A4, Dako, Copenhagen, Denmark) recognizing all p63 isoforms and a monoclonal antibody directed against p53 (DO7, Dako, Copenhagen, Denmark) were used. Antibodies were used at 1:50, and staining performed using a Ventana staining machine (Ventana medical systems, Tucson, Arizona, USA) and reagents according to the supplier’s recommendation.
Statistical analysis
All analyses were carried out using SPSS statistics 17.0. The relative fold change expression values for all mRNA and miRNAs displayed a non-normal distribution and data was thus log-transformed to create a normal distribution for parametric analysis. Paired-sample t-tests were conducted to evaluate the effect of NB-UVB phototherapy on gene expression following treatment. Independent-sample t-tests were used to compare gene expression in psoriasis vs. healthy controls and Pearson’s coefficient correlation was used for correlation analysis. In all statistical tests, two-tailed p-values < 0.05 were considered statistically significant.
Results
Effect of NB-UVB phototherapy on p63 expression in psoriatic epidermis
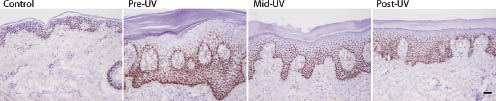
In accordance with our previous results (31), TAp63 mRNA levels were significantly down-regulated in psoriasis (pre-UV) compared with healthy controls, with a mean fold change of –7.49 (p < 0.001) (Fig. 1A). Following NB-UVB phototherapy, TAp63 expression in post-UV epidermis was up-regulated; however, no significant difference was found when comparing that with TAp63 expression in pre-UV or mid-UV epidermis. ΔNp63 transcripts were also significantly down-regulated in psoriasis compared with healthy controls (mean fold change –2.08, p < 0.001) and remained low following phototherapy (Fig. 1A). Immunohistochemical staining using an antibody recognizing all p63 isoforms showed p63 expression to be similar before, during and after NB-UVB phototherapy, with p63 expressing cells seen almost throughout the whole epithelium, with the strongest expression in basal and suprabasal layers (Fig. 2).
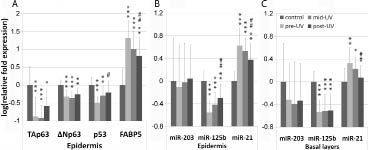
Fig. 1. Gene expression in healthy untreated skin (control) and psoriatic lesions prior to (pre-ultraviolet (pre-UV)), during (mid-UV) and at the last stage of narrow-band ultraviolet B (NB-UVB) phototherapy (post-UV). (A) Expression of TAp63, ∆Np63, p53 and FABP5 in epidermis. (B) Expression of miR-203, miR-125b and miR-21 in epidermis. (C) miRNA levels in the basal layers of epidermis. The pool set of data from 12 controls was set to 1 and log-transformed. The mean and SD of log-transformed relative mRNA fold expression were shown. Independent sample t-test was performed for comparing psoriasis with controls (***p < 0.001, **p < 0.01, *p < 0.05) and a paired-sample t-test was performed for comparing gene expression in post-UV with pre-UV psoriatic lesions (##p < 0.01, #p < 0.05). One patient stopped treatment halfway, and only 11 samples are thus included in the post-UV group.
Fig. 2. Immunohistochemical staining of p63 in skin sections from controls and psoriatic lesions following narrow-band ultraviolet B (NB-UVB) phototherapy. Similar p63 expression pattern was found in control and psoriasis lesions prior (pre-ultraviolet (pre-UV)), at the middle stage (mid-UV) and before the last session of phototherapy (post-UV). p63 was expressed almost throughout the whole epithelium, with the strongest reaction seen in the basal and suprabasal cell layers. Fewer p63-expressing cells were seen in the most differentiated cell layers. Scale bar: 50 µm.
For comparison with p63, we also examined expression of p53 in this patient group. Only occasional p53-expressing cells were detected in normal skin by immunostaining, and in accordance with previous findings, p53 protein expression was increased in psoriatic lesions and the expression was further increased following NB-UVB phototherapy (data not shown). p53 mRNA levels were, however, significantly down-regulated in psoriasis compared with healthy controls (mean fold change –3.08, p < 0.001). When comparing p53 expression in post-UV with pre-UV lesions, a significant increase was found (p = 0.02). Notably, no significant difference was seen when comparing post-UV lesions with healthy controls regarding p53 mRNA expression (Fig. 1A). FABP5, the positive control used in this study, was, as expected, significantly up-regulated in psoriasis (mean fold change 20.97, p < 0.001) as well as significantly down-regulated after treatment (p = 0.01) (Fig. 1A).
Effect of NB-UVB phototherapy on miRNA in psoriatic epidermis
Expression of miR-203, miR-125b and miR-21 was examined in psoriatic and normal epidermis showing significant down-regulation of miR-125b and significant up-regulation of miR-21 in psoriasis (p < 0.001). For miR-203 no significant change was seen in psoriatic compared with normal epidermis. NB-UVB phototherapy significantly increased miR-125b expression (p = 0.003) and also significantly decreased miR-21 expression (p = 0.003), whereas no significant change in miR-203 expression was seen (Fig. 1B). When looking at the most basal layers only, no significant effect on miR-125b was seen, whereas expression of miR-21 was down-regulated (p = 0.002) following phototherapy. No significant difference in miR-21 expression was seen in post-UV lesions compared with healthy controls (Fig. 1C).
Association between p63 and miRNAs
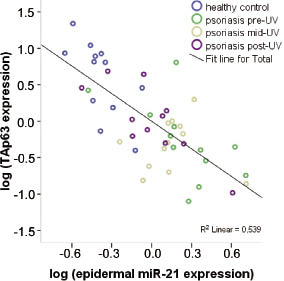
Since miR-21 is a negative regulator of TAp63 (29), levels of epidermal miR-21 expression were plotted against levels of TAp63 from all healthy and psoriasis samples (n = 47). Using Pearson’s correlation analysis a significant correlation between expression of TAp63 and miR-21 was seen (r = –0.734, p < 0.001). When analysing data from healthy controls and pre-UV samples only (n = 24), a higher correlation between TAp63 and miR-21 was found. Analysis of data from mid-UV and post-UV samples (n = 23), however, showed a weaker correlation between TAp63 and miR-21 (r = –0.555, p = 0.006).
Association between clinical response to NB-UVB phototherapy and gene expression
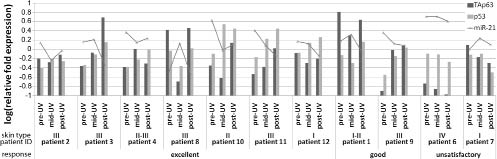
Response assessment on the 11 patients who fulfilled treatment showed 7 patients with excellent clinical response, 2 with good response and 2 with unsatisfactory response. All patients with excellent and good response to treatment (9 patients) showed up-regulation of p53 at both mRNA (Fig. 3) and protein level. In 2 patients with unsatisfactory response, decreased p53 mRNA levels were seen as well as no obvious change in protein accumulation following treatment. p53 expression thus appears to be connected to clinical response. Expression of TAp63, ∆Np63, FABP5 and miRNAs was also analysed according to patient response, but no obvious correlation was found (Fig. 4).
Fig. 3. Correlation of miR-21 and TAp63 mRNA levels in epidermis. Scatter plots showed that the increase in TAp63 expression was inversely correlated with increased levels of miR-21. Pearson’s correlation test was used to assess the association (n = 47, p < 0.001).
Fig. 4. TAp63, p53 and miR-21 expression in psoriatic epidermis following narrow-band ultraviolet B (NB-UVB) phototherapy. Eleven patients who fulfilled treatment were assessed and grouped into three categories: excellent (n = 7), good (n = 2) and unsatisfactory (n = 2) response. Gene expression levels of TAp63, p53 and miR-21 in psoriatic epidermis before (pre-ultraviolet (pre-UV)), during (mid-UV) and after phototherapy (post-UV) are shown. Log-transformed fold expression relative to the pool set of data from 12 untreated healthy controls are shown.
Discussion
NB-UVB phototherapy is an effective treatment for most patients with psoriasis, and in this study, 9 of 11 patients responded satisfactorily. In NB-UVB treated epidermis, several psoriasis-related molecules were significantly modified, such as p53, FABP5, miR-21 and miR-125b, whereas neither TAp63 nor ∆Np63 was significantly affected. p63 is a key molecule for maintenance of epithelial homeostasis and in addition to regulating genes with roles in keratinocyte survival, proliferation, differentiation and adhesion, it controls certain chemokine ligands in human epithelial cells, indicating its role in epithelial immune surveillance (34). We have previously shown that TAp63 mRNA was significantly down-regulated in psoriatic lesions compared with healthy controls, and a trend towards decreased TAp63 expression was also seen in patients’ clinically normal skin. Now we found that despite clinical improvement, p63 status was not significantly affected by NB-UVB phototherapy. As psoriasis patients show relapse within shorter or longer intervals, this remaining defect in p63 status could be a causative factor not only for initiation but also for maintenance of the disease.
Similar to Jasim et al. (11), we saw increased expression of p53 protein in untreated psoriasis samples compared with controls, and a further increase during treatment. p53 mRNA on the other hand was significantly down-regulated in psoriasis, and levels significantly increased following treatment. The therapeutic effect of NB-UVB on psoriasis thus seems to evoke p53 both at mRNA and protein level. Following NB-UVB phototherapy, increased p53 expression was seen in patients with better clinical response, with the increased accumulation of p53 mainly seen in the basal and suprabasal layers of psoriatic epidermis. However, in patients with unsatisfactory clinical response to phototherapy, decreased p53 mRNA expression was observed and less p53 positive cells found in the basal or suprabasal layers. p53 status therefore seems correlated to patient’s clinical response, suggesting that p53, when satisfactory clinical response is achieved, participates in proliferative regulation of keratinocytes. As the present material was limited to only 11 patients, further investigation with a larger group of patients is needed in order to clarify the relationship between gene expression and patient response.
miR-21 is an important oncogene targeting a network of p53, transforming growth factor (TGF)-beta and mitochondrial apoptosis tumour suppressor genes in glioblastoma cells (29). Up-regulation of miR-21 has been shown in psoriasis. Here, we found that epidermal miR-21 was significantly down-regulated following phototherapy. Furthermore, by examining miRNA expression in the basal layers of epidermis only, we found that miR-21 was also successfully targeted here. As miR-21 also is involved in cell cycle arrest and apoptosis pathways, our results highlighted the importance of controlling miR-21 concerning effect on basal cell proliferation.
miR-21 can negatively regulate TAp63 mRNA and proteins levels in glioblastoma cells. Overall, we found a reverse correlation between miR-21 and TAp63 mRNA levels, which was particularly strong in controls and pre-UV samples. In addition, using a commercially available antibody recognizing all p63 isoforms, we confirmed the similar expression pattern of p63 previously seen in psoriasis and normal epidermis (34). These results indicate a complex mechanism in TAp63 regulation in vivo, which merits further investigation.
miR-125b is a known negative regulator of p53 proteins (30); however, no obvious correlation between them was seen in vivo. Levels of both miR-125b and p53 proteins were up-regulated following phototherapy. Most p53-positive cells were found in the basal layers where levels of miR-125b remained unaffected. These findings indicate that miR-125b might not correlate to p53-induced apoptosis in psoriasis. Nevertheless, deregulation of miR-125b might account for other important phases in psoriasis, such as aberrant cytokine signalling and inflammation (35). In contrast to results from other groups (25, 26), we did not find any significant difference in expression of miR-203, a regulator of ∆Np63, in psoriasis compared with normal controls (35). An explanation for this could be the individual variation in miR-203 expression that we saw, the use of different techniques, or the fact that we studied epidermis only.
In conclusion, in a group of patients with psoriasis, we have shown significant changes in expression of miR-21 and miR-125b following treatment with NB-UVB phototherapy, indicating important roles for these molecules in treatment of the disease. Data also suggest an important role for p63 in the pathogenesis of psoriasis, and that targeting p63 effectively in psoriatic epidermis may be essential in order to achieve better long-term clinical improvement.
Acknowledgements
This study was supported by grants from Lion’s Cancer Research Foundation, Umeå University, and The Swedish Cancer Society contract number 10 0324. We are grateful to Maria Östman for her help with patient recruitment and collection of biopsies. We also gratefully acknowledge the skilful technical support provided by Astrid Höglund.
References