Chiara Cortelazzi1, Nicoletta Campanini2, Roberto Ricci2 and Giuseppe De Panfilis1
Departments of 1Dermatology and 2Pathology, Via Gramsci 14, IT-43126 Parma, Italy. E-mail: chiara.cortelazzi@alice.it
Accepted April 3, 2012.
Chiara Cortelazzi1, Nicoletta Campanini2, Roberto Ricci2 and Giuseppe De Panfilis1
Departments of 1Dermatology and 2Pathology, Via Gramsci 14, IT-43126 Parma, Italy. E-mail: chiara.cortelazzi@alice.it
Accepted April 3, 2012.
Various effector CD4+ T-lymphocyte subsets have been characterized, such as T-helper (Th)1, Th2, regulatory CD4+ T cells (Treg), Th17, and Th22, which show distinct patterns of cytokine release. Specifically, the cytokine interleukin (IL)-9 has been regarded largely as an exclusive Th2 cytokine. However, a new subset of the Th population, named “Th9”, which is separate from Th2, producing IL-9 in large quantities has been described (1, 2). More recently, new information concerning Th9 cells confirmed their uniqueness with regard to the unusual production kinetics of IL-9 and the short retention of these cells in affected target tissues (3).
For the generation of Th subpopulations, transcription factors, formerly believed to be specific of each subset, are required, such as Tbet for Th1 cells, GATA-3 for Th2 cells, Foxp3 for Treg, and ROR-γt for Th17 cells. A recent publication (4) further assessed the Th9 identity by showing that the transcription factor PU.1 is required for the development of Th9 lineage; indeed, PU.1 binds directly to the IL-9 promoter to trigger specific chromatin modifications (5).
Since PU.1 was formerly known to be expressed in myeloid lineage and B lymphocytes, and to be required for normal macrophage and granulocyte differentiation, the utility of PU.1 as an immunohistochemical marker for skin was restricted to primary skin neoplasms of non-lymphoid cell origin (6). To the best of our knowledge, PU.1 expression has never been investigated to identify Th9 cells within cutaneous lesions.
The aim of the present study was therefore to investigate whether CD4+ PU.1+ cells (4, 5) are recognizable in skin sections, specifically within some inflammatory cutaneous infiltrates.
MATERIALS AND METHODS
Ten formalin-fixed, paraffin-embedded skin samples, previously collected at the time of biopsy, were utilized: they included 4 cases of chronic lesions of atopic dermatitis (AD), 3 cases of chronic plaque psoriasis, and 3 cases of chronic lesions of allergic contact dermatitis (ACD) to nickel. For PU.1 staining, the anti-PU.1 antibody “G148-74, ab 48587” (Abcam, Cambridge, UK) was used, and for IL-9 staining the anti-IL-9 antibody “ab111915” (Abcam) was used. A previously described technique was carried out for single-labelling immunocytochemistry (7). Similarly, the anti-CD3 antibody “anti-CD3 mAb” (Neomarkers, Fremont, CA, USA) and the same technique were used to reveal CD3+ cells.
In order to reveal CD4+ PU.1+ cells (4, 5), a double-staining immunocytochemical technique was performed (7).
Negative controls for PU.1 antibody included avoiding the antibody itself, or using an unlabelled anti-mouse antibody after the antibody; for a further control, normal skin samples from healthy donors were also used. In addition, a negative control for the anti-IL-9 antibody was to use the isotype control antibody ab27472 (Abcam) instead of the anti-IL-9 antibody. Positive control was to reveal PU.1+ cells within sections of reticulohistiocytoma and juvenile xanthogranuloma (6).
Quantitative evaluation of positively stained cells was performed by cell analysis of PU.1-infiltrated inflammatory environments, as described (7).
RESULTS
PU.1 was detected immunohistochemically at the single-cell level by brown, mainly nuclear, staining (Fig. 1). Indeed, numerous stained cells were detectable within the mostly lymphocytic microenvironment of AD lesions (Fig. 1a), and scattered stained cells were detectable in psoriatic lesions (Fig. S1a; available from http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1408), whereas few stained lymphocytes were detectable within the lesions of ACD (Fig. S1b). IL-9 was detected on serial sections of AD, psoriasis and ACD by weak cytoplasmic staining (Fig. 1a, inset), whereas the cell periphery was stained by the anti-CD3 antibody (Fig. S1b, inset).
Double-staining experiments showed that some PU.1+ cells (brown nuclear staining) co-expressed CD4 (red peripheral staining) (Fig. 1d).
Controls for the PU.1 antibody and for the anti-IL-9 antibody gave negative results, and normal skin samples from healthy donors were negative (Fig. 1c). Strong staining for PU.1 was seen in histiocyte-derived tumours (Fig. 1b), as expected (6).
Quantitative evaluation of positively stained cells showed that the percentage of PU.1+ cells was 23.4% of total lymphocytes in AD, 17.3% in psoriasis, and 5.8% in ACD.
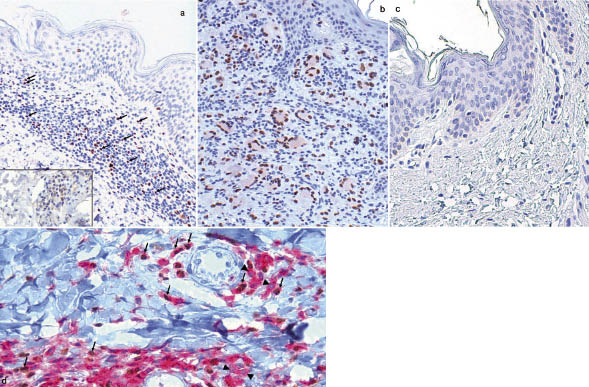
Fig. 1. (a) Numerous lymphocytes in a atopic dermatitis lesion show brown nuclear staining for PU.1 (arrows) (× 20). Inset: Dermal cells in a serial section show cytoplasmic staining for IL-9 (× 40). (b) Positive control. Numerous cells in the mainly histiocytic environment of juvenile xanthogranuloma show evident brown staining for PU.1 (× 20). (c) Negative control. Normal skin is PU.1– (× 20). (d) Some cells in the context of a lesion of a patient with atopic dermatitis are double-labelled (arrows); namely, show both CD4+ (red peripheral staining) and PU.1 positivity (brown nuclear staining). Other CD4+ cells are PU.1– (arrowheads), whereas the unlabelled cells are CD4+ PU.1– (original magnification × 40).
DISCUSSION
This study provides early evidence of the detection of CD4+ PU.1+ cells, i.e. putative Th9 lymphocytes (1–5), within the cellular infiltrate of some inflammatory cutaneous diseases. It is still difficult to conclusively establish the identity of Th9 cells, because IL-9 may be produced, in limited proportions (4), also by other Th subsets (reviewed in 8). Under certain cytokine conditions even committed Th2 cells can be “reprogrammed” toward a Th9 phenotype (1). This suggests that there is plasticity between Th2 and Th9 subsets (5), and one factor that may promote the switch between these phenotypes is the transcription factor PU.1 (4). Even activation of naive CD4+ T cells in the presence of IL-4 and transforming growth factor beta (TGFβ) significantly enhance IL-9 production (1, 2).
Reportedly, Th9 cells are required for allergic inflammation and immunity to intestinal parasites (1, 4), and can play a role in allergic inflammation by virtue of the pleiotropic functions of IL-9 (8), and thus overall contribute to allergic diseases (9). Moreover, IL-9 expression is high in lungs of asthmatic patients (10), and blocking anti-IL-9 antibodies have been developed as a therapy for atopic diseases (11). Therefore, although there are no published reports regarding the involvement of IL-9 with AD (9), the present detection of Th9 cells within the AD infiltrate is not surprising.
Although CD4+ cells represent the most prominent cell type infiltrating the dermis of psoriatic lesions (12), our finding of scattered Th9 cells was somehow unexpected, because the overwhelming Th subsets within psoriatic lesions are Th1 and Th17. On the other hand, multiple activated cell types in the lesional psoriatic skin are responsive to IL-9, including T cells, antigen-presenting cells and keratinocytes (8). Even more interestingly, IL-9 enhances the proliferation and/or accumulation of Th17 cells (13), and is a mediator of Th17-driven inflammatory diseases (reviewed in 14).
ACD is mostly due to the rapid recruitment of chemical-specific CD8+ T cells, which induce apoptosis of keratinocytes; in addition, Th1 and Th17 cells contribute to the extension of the inflammatory reaction (reviewed in 15). Thus, the scarcity of Th9 cells in the lesions of ACD, herein demonstrated, was not surprising.
It is tempting to hypothesize that, as suggested for general diseases (9), Th9 cells may contribute to the pathophysiology of different cutaneous diseases, particularly by orchestrating a broad repertoire of cytokines. In fact, recent studies have revealed that IL-9 promotes inflammation (10, 14) and allergic functions (9) mainly by recruiting macrophages, mast cells and eosinophils (1, 10, 14). On the other hand, IL-9 modulates virus-initiated inflammation, and even enhances the immunosuppressive activity of natural Treg (13). Thus, Th9 cells in the skin might also play the recently envisioned role of regulators of pathogenic vs. protective mechanisms of immune responses.
REFERENCES
