Nanako Niiyama1, Takeshi Sasahara2, Hiroshi Mase3, Michiko Abe4, Haruo Saito5 and Kensei Katsuoka1
Departments of 1Dermatology and 2Microbiology, Kitasato University School of Medicine, Kanagawa, 3New Materials Development Center, Mitsui Chemicals Inc. (currently: Mitsui Chemical Analysis & Consulting Service Inc.), Chiba, 4Faculty of Hygienic Technology, Kitasato University School of Allied Health Sciences, Kanagawa, and 5Japan Copper Development Association, Tokyo, Japan
Metallic copper has been shown significantly to reduce methicillin-resistant Staphylococcus aureus (MRSA) contamination of the ambient surroundings of the beds of MRSA-carrying patients in dermatology wards. The aim of this study was to determine whether a bed sheet made of copper-coated film will reduce the spread of MRSA contamination in the environment of a heavily-colonized patient. The bacterial count was highest on the bed sheet. MRSA cell counts on the surface of the non-film-coated control sheet were high (6,600–11,000 colony forming units (cfu)), but those on the copper film were considerably lower (20–130 cfu). Use of metallic copper on the bed sheets of patients who are likely to be a source of MRSA contamination may help to prevent the spread of MRSA contamination in hospital wards. Key words: copper; Pseudomonas aeruginosa; Staphylococcus aureus.
Accepted Jun 26, 2012; Epub ahead of print Oct 5, 2012
Acta Derm Venereol 2012; 92: XX–XX.
Nanako Niiyama, Department of Dermatology, Kitasato University School of Medicine, 1-15-1 Kitasato, Minami-ku, Sagamihara, Kanagawa, 252-0374 Japan. E-mail: dermananako@ad.cyberhome.ne.jp
Methicillin-resistant Staphylococcus aureus (MRSA) causes healthcare-associated infections, and accounts for approximately 60% of all S. aureus strains isolated from clinical sites in Japan (1). In recent years, there has been a trend towards an increase in the rate of isolation of MRSA from patients with suppurative skin or soft tissue diseases, such as furuncles and contagious impetigo, in the community (2). These MRSA isolates, unlike nosocomial MRSA isolates, are characterized by their ability to produce a leukocyte-destroying toxin called Panton-Valentine leukocidin (PVL), and by the presence in their genome of a type V methicillin-resistant gene region. The only effective method to prevent transmission of nosocomial infection with these MRSA strains is to block contact transmission via infectious material from MRSA-infected patients/carriers, such as pus, sputum, stools, etc., or via the ambient surroundings of the patients, including instruments and materials coming into frequent contact with MRSA-infected patients or carriers. Although standard preventive measures, such as hand-washing by healthcare workers, wearing of gowns and gloves, and antiseptic cleaning of the contaminated environmental surface are already in practice, further enhancement of preventive measures is required.
Contamination rates of the hospital environment with MRSA from MRSA carriers or infected patients in the hospital are particularly high when the patients show colonization or infection of wounds or the urinary tract by MRSA, exceeding 35% for the floor around the patient’s bed, bed linen, and gowns (3, 4). In addition, MRSA contamination is frequent in indoor environments, including bathrooms and ointment treatment rooms in the dermatology ward (5). The surroundings of inpatients with exfoliative dermatitis represent a risk factor for nosocomial infection with MRSA (6, 7). However, it is unclear how MRSA contamination spreads in the ambient surroundings of the beds of inpatients.
Copper and copper alloys have the potential to kill pathogenic microorganisms, including MRSA, that come into contact with the surface of the metal (8–11), and the US Environmental Protection Agency (EPA) acknowledged, in February 2008, that that only copper alloys containing 60% or more copper exert significant bactericidal activity (12). Therefore, attempts to use copper alloys for the control of nosocomial infections have been initiated worldwide (13). In Japan, metallic copper has already been shown significantly to reduce MRSA contamination of the ambient surroundings of the beds of MRSA-carrying patients in dermatology wards. It has been suggested that copper alloys may facilitate reduction in the risk of MRSA contamination, and may play an effective role in the prevention of the spread of nosocomial MRSA infection (14).
The aim of this study was to determine the status and spread of MRSA contamination in the surroundings of an infected patient, an MRSA-infected carrier who was admitted to the dermatology ward of Kitasato University Hospital with cutaneous malignant lymphoma, and who had systemic erythema accompanied by desquamation, i.e. erythroderma. In addition, the ability of copper to prevent the spread of MRSA contamination from the patient to the surroundings was examined by applying a copper-coated film to the patient’s bed linen.
MATERIALS AND METHODS
Copper-coated film and its usage
Copper-coated film (copper film) is a polyethylene terephthalate film product coated with a thin layer of copper, less than 1/10,000 mm thick, developed by New Materials Development Center, Mitsui Chemicals Inc (Chiba, Japan). The film was cut appropriately for use in the disinfection performance test (21 × 39,7 cm) and evaluation of the ward environment.
For evaluation of the ward environment, the copper film was fixed to the bed sheets of the MRSA-carrying/infected patient (in areas corresponding to the patient’s shoulders, back and lumbar region) with double-sided adhesive tape (Fig. 1). Fixation of the film was carried out when the bed sheets were changed. The patient’s bed sheets were changed every day, and the bed linen, including the contaminated sheets, were carefully folded inwards, placed in a hermetically-closed, water-soluble laundry bag, and washed with hot water. The surroundings, materials and instruments, were cleaned with rubbing alcohol every morning and evening. The floor was wiped with a dry ultrafibre cloth twice a day (in the morning and evening).
Fig. 1. Copper film attached to a bed sheet. During the study copper film was attached in 3 locations.
Testing the disinfection performance of the copper film
For the disinfection performance test, the following 4 strains were used: patient skin-derived MRSA (patient MRSA strain), MRSA ATCC 43300 (standard MRSA strain), S. aureus NBRC 13275 (standard SA strain), and Pseudomonas aeruginosa NBRC 12732 (standard PA strain). Before the test, each strain was inoculated onto nutrient agar medium (Nissui Pharmaceutical Co. Ltd, Tokyo, Japan), incubated overnight at 36ºC, and suspended in physiological saline to obtain a suspension with a bacterial density of 2 × 106 colony forming units (cfu)/ml.
The disinfection performance test of a copper plate (Japan Industrial Standard: JIS C1220) and copper film was carried out according to a previously reported film adhesion method (JIS Z2801) (9, 11). Specifically, a 100 ml aliquot of the bacterial suspension (105 cfu) was inoculated onto the copper test plate (3 × 3 cm), covered with a piece of polyethylene film (2 × 2 cm), and the reaction allowed to proceed for up to 3 h at 36ºC (experimental group). Controls were prepared with a piece of polyethylene film (3 × 3 cm) (control group). In some experiments, after inoculation of the bacterial suspension onto the copper plate, 3.6 mM bathocuproine disulphonic acid, disodium salt (BPSA, specific chelator of Cu2+, Dojindo Molecular Technologies, Inc., Kumamoto, Japan), 5 mM ethylenediaminetetraacetic acid (EDTA, chelator of Cu2+, Dojindo Molecular Technologies, Inc., Kumamoto, Japan), 200 mM D-mannitol (Sigma-Aldrich, Tokyo, Japan), 300 units superoxide dismutase (SOD, Sigma-Aldrich, Tokyo, Japan), or 300 units catalase (Sigma-Aldrich, Tokyo, Japan) was added, and the plate was covered with a piece of film to allow the reaction to proceed as above. The copper coupons in experimental group and the pieces of polyethylene films in control group were collected at specific time, placed in 10 ml of Soybean-casein-digest-lecithin-polysorbate broth medium (Eiken Chemical Co. Ltd, Tokyo, Japan) and vortexed vigorously. Serial 10-fold dilutions of the media was prepared to 10-2 in physiological saline. Appropriate dilutions of each medium were plated on standard agar medium (Nissui Pharmaceutical Co. Ltd, Tokyo, Japan). The plate was incubated at 36˚C for 48 h. Colonies on the plate were counted and multiplied by 10, and the results were expressed as the average number of cfu ± standard deviation (SD) in triplicate (cfu/4 cm2).
Patient and test sample
The subject was a 47-year-old woman with cutaneous malignant lymphoma, who was hospitalized between June 13, 2008 and April 1, 2009 in the dermatology ward of Kitasato University Hospital. Her sputum and skin swab culture revealed MRSA. The major antibacterial drugs she was treated with during her hospital stay were linezolid (LZD), doripenem (DPRM) and pazufloxacin (PZFX).
Her bed sheets were examined for bacterial contamination, including MRSA, between 24 June 2008 and 16 January 2009. To determine the spread of MRSA contamination to the surroundings of the patient, the over-bed table, bedside table, bed rail, top panel of the air conditioner, wash stand, mobile phone, plastic gloves used for treatment, and the floor were examined on June 25, 2008. Evaluation of the disinfection performance of the copper film on the bed sheet was carried out during the period June 26 to July 16, 2008. The room temperature and humidity during this period were maintained at 26–27ºC and 55–63%, respectively.
This study was performed with strict adherence to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients. Institutional Review Board approval was obtained.
Bacteriological examination
Test samples were collected by the stamp method using contact plates (Eiken Chemical Co. Ltd, Tokyo, Japan) and by a wet-wiping method using 3M quick swab (3M Microbiology, Minnesota, USA). Samples from the surfaces of bed sheets and copper films were obtained by the stamp method, and samples from environmental surfaces other than bed sheets were obtained by the wiping method using a 100 cm2 frame. Triplicate samples were prepared for each test. Egg yolk-enriched mannitol salt agar (MSEY medium, Eiken Chemical Co. Ltd, Tokyo, Japan) was used for the isolation culture of Staphylococcus species, including MRSA. MDRS II medium containing oxacillin (MPIPC) and ceftizoxime (CAZ) (Kyokuto Pharmaceutical Industrial Co. Ltd, Tokyo, Japan) was used for confirmation of the MRSA, and NAC agar medium (Eiken Chemical Co. Ltd, Tokyo, Japan) for isolation culture of P. aeruginosa. Each culture was continued for 48 h at 36ºC. The cfu values were calculated for MRSA, other Staphylococcus spp., and P. aeruginosa. The mean cfu value was obtained for each set of triplicate samples, and expressed as the mean viable cell count per unit area ± SD (cfu/25 cm2 or cfu/100 cm2).
MRSA was identified by a polymerase chain reaction (PCR) technique (15), using mecA gene-specific primers (mecAF: 5-agattgggatcatagcgtca-3 and mecAR: 5-gaaggtatcatcttgtaccc-3) designed based on the GenBank registry database AB236888 (S. aureus mecA gene for penicillin-binding protein 2’). PVL was identified by a PCR technique (16) using lukPV gene-specific primers (lukPVF: 5-atcattaggtaaaatgtctggacatgatcca-3 and lukPVR: 5gcatcaastgtattggatagcaaaagc-3). The presence or absence of PCR products of the mecA gene and lukPV gene (259 bp and 433 bp) was confirmed by agarose gel electrophoresis.
Pulse-field gel electrophoresis
DNA genotypes of MRSA strains isolated from the surroundings of the patient were analysed by pulse-field gel electrophoresis, according to the procedures reported previously (17). Specifically, bacterial cell bodies included in the agarose were treated with lysozyme (Sigma-Aldrich, Tokyo, Japan) and proteinase K (Sigma-Aldrich, Tokyo, Japan) to extract chromosomal DNA. The chromosome DNA was digested wtih the restricted enzyme XbaI (Takara Bio Inc., Shiga, Japan), and separated by a contour-clamped homogeneous electric field electrophoresis (CHEF Mapper; Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
The bactericidal effect of the copper film was analysed with Wilcoxon and Mann–Whitney t tests, with the significance level set at p < 0.05.
RESULTS
Status of Methicillin-resistant Staphylococcus aureus contamination of the patient’s surroundings
The status of contamination of the patient’s bed sheets with Staphylococcus spp., including MRSA, was determined. The results are shown in Fig. 2. Numerous MRSA cells, up to 6,300 to 10,000 cfu, and other Staphylococcus spp. cells, up to 3,000–7500 cfu, were detected on June 24–27, 2008. Thereafter, administration of antibacterial drugs, such as LZD and DRPM, led to alternate detection of contamination with Staphylococcus spp., including MRSA or P. aeruginosa. Namely, the contaminating cell count of MRSA and other Staphylococcus spp. began to decrease rapidly immediately after administration of LZD, and reached nil or minimum, if any, on July 1. Three days later, P. aeruginosa at 5,000 cfu was detected because of microbial substitution; thereafter, the P. aeruginosa cell count declined markedly after administration of DRPM, and MRSA appeared again on July 16, at a cell count as high as 10,000 cfu, on July 28 (Fig. 2).
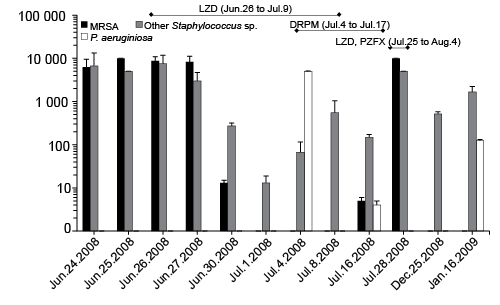
Fig. 2. Status of contamination of the patient’s bed sheets by Staphylococcus spp., including Methicillin-resistant S. aureus (MRSA) and P. aeruginosa. LZD: linezolid; DRPM: doripenem; PZFX: pazufloxacin.
The pattern of transmission of MRSA to the environment surrounding the patient’s bed was examined on July 25, 2008, when MRSA contamination of the bed sheet began to increase again. The results revealed MRSA contamination of the bed rail, over-bed table, mobile phone, bedside table, and wash stand, in descending order of cell count. The MRSA cell count was proportional to the frequency of contact of the examined article with the patient. High MRSA cell counts were also found on gloves used for the patient’s treatment and on the floor around the bed (Table I).
Table I. Status of contamination of the surroundings of the patient’s bed by Staphylococcus sp., including Methicillin-resistant Staphylococcus aureus (MRSA)
|
|
Bacteria, n (cfu/100 cm2) |
|
|
MRSA |
Other Staphylococcus sp. |
|
|
Bed linens |
40,000 ± 0 |
8,000 ± 400 |
|
Over-bed table |
138 ± 90 |
218 ± 10 |
|
Bedside table |
10 ± 0 |
42 ± 35 |
|
Bedside rail |
6,320 ± 500 |
1,250 ± 300 |
|
Top panel of the air conditioner |
Not detected |
Not detected |
|
Wash stand |
8 ± 2 |
16 ± 10 |
|
Mobile phone |
128 ± 20 |
83 ± 14 |
|
Disposable gloves (used) |
4,440 ± 1593 |
1,250 ± 0 |
|
Disposable apron (used) |
6 ± 4 |
6 ± 4 |
|
Floor |
379 ± 196 |
1,663 ± 408 |
Analysis of the MRSA DNA genotype
Chromosomal DNA analysis was carried out for MRSA strains isolated from the surrounding environment and the skin of the patient from June 24 to July 25, 2008, and their homology was determined. The results revealed that all MRSA strains isolated from the bed sheets and the skin of the MRSA-carrying/infected patient had 98% or higher homology, demonstrating that they had the same DNA genotype (Fig. 3A). It is noteworthy that the MRSA strains detected again after having been eliminated once through microbial substitution following antibiotic therapy had the same DNA genotype as that of the MRSA strains detected before the microbial substitution (lanes 7, 8 of Fig. 3A).
The MRSA strains isolated on 25 July from the patient’s skin and her surroundings, including her bed sheets, also showed 98% or higher homology. These findings indicate that MRSA strains that colonized the skin and those contaminating the surrounding environment had the same DNA genotype (Fig. 3B). Thus, it is inferred that MRSA strains isolated from the surroundings of the patient were derived from direct contact with the patient’s hands and the gloves of the healthcare workers, and exfoliation of the scaly skin lesions.
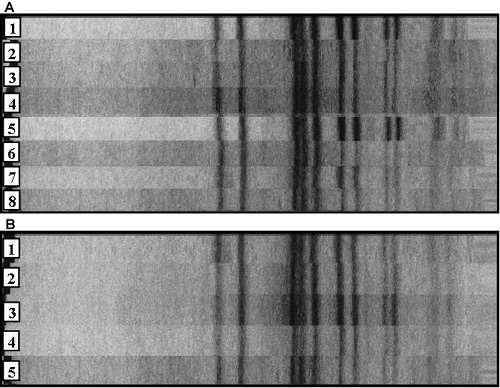
Fig. 3. DNA genotype analysis of Methicillin-resistant S. aureus (MRSA) strains isolated from the patient’s skin and bed sheet between June 24 and July 25, 2008. (A) shows that MRSA which was transmitted again after having disappeared once is genetically the same as MRSA detected early in infection. Lanes 1 and 8: MRSA strains isolated from the patient skin on 24 June and 25 July 2008, respectively. Lanes 2–7: MRSA strains isolated from the bed sheet on June 24, June 26, June 27, June 30, July 15 and July 25, 2008, respectively. (B) shows that MRSA isolated from the surroundings of the patient is also the same as MRSA from the patient. Lane 1: MRSA strain isolated from the patient skin on July 25, 2008. Lanes 2–5: MRSA strains isolated from mobile phone, bedside table, bedside rail and disposable gloves (used) on July 25, 2008.
Bactericidal effect of copper film
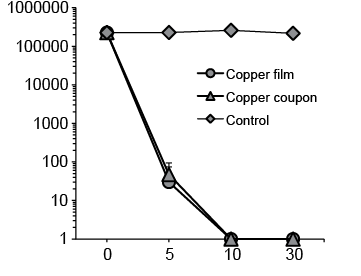
Whether copper film might also exert a bactericidal effect on MRSA and P. aeruginosa, like metallic copper, was examined using the patient MRSA, standard MRSA, standard S. aureus, and standard PA strains. The results revealed that, similar to copper plates, the copper film caused a gradual decrease in the viable cell count of each strain in proportion to the length of the reaction time. Patient and standard MRSA strains and the standard S. aureus strain were killed completely within 180 min (Fig. 4), and the standard PA strain within 10 min (Fig. 5).
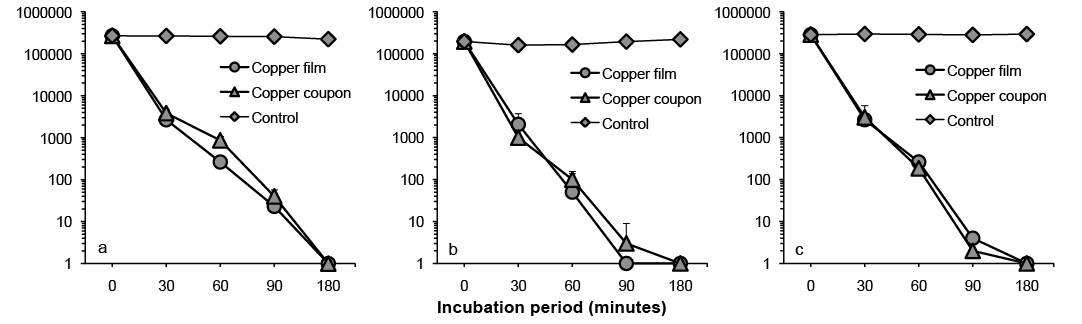
Fig. 4. Bactericidal effects of copper on (a) standard Staphylococcus aureus, (b) standard Methicillin-resistant S. aureus (MRSA), and (c) patient MRSA strains.
Fig. 5. Bactericidal effect of copper film on Pseudomonas aeruginosa NBRC 12732.
To elucidate the bactericidal mechanism of the copper surface against S. aureus, the influences of several reagents (the metal chelators EDTA and BPSA, the hydroxyl radical remover mannitol, the superoxide remover SOD, and the hydrogen peroxide-degrading enzyme catalase) on the bactericidal effect of metallic copper against standard S. aureus strains were examined. The results are shown in Fig. 6. The bactericidal effect of copper was annulled over time by EDTA and BPSA. This suggests that the presence of ionized Cu+ and Cu2+ on the copper surface (particularly Cu+) is indispensable for the bactericidal effect of the copper surface. The bactericidal effect of copper was also eliminated by mannitol, SOD and catalase, and these findings show that the reactive oxygen species (ROS), including hydroxyl radicals, superoxide and hydrogen peroxide produced as a result of ionization via oxidation of the copper surface, was involved.

Fig. 6. Results of the disinfection performance test of copper plates in the presence of ethylenediaminetetraacetic acid (EDTA), bathocuproine disulphonic acid, disodium salt (BPSA), mannitol, superoxide dismutase (SOD) and catalase.
Preventive effect of the copper film on the spread of MRSA contamination of the environment
The copper film was attached to the bed sheets of the MRSA carrier, where contamination with MRSA and other Staphylococcus spp. was prominent, and the possible preventive effect of copper on the spread of MRSA contamination from the MRSA carrier to the surrounding environment was examined during a period of approximately 1 month from June 26 to July 16, 2008. The results are shown in Table SI (available from http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1472). The cell counts of MRSA and other Staphylococcus spp. on the copper film surface were considerably lower than those on the non-film-coated control sheet surface. Although contamination with P. aeruginosa was confirmed on July 4 and 16, hardly any P. aeruginosa was detected on the copper film surface.
DISCUSSION
MRSA has been detected in various environments in the hospital. Contamination vehicles reported frequently include the indoor floors, indoor air, door handles, and bed linen (18). In the event of development of nosocomial MRSA infections in patients, MRSA is frequently isolated from the surrounding environment, the frequency of isolation from indoor floors being second only to that from the linen and gowns of the patients (3, 4). In this study, contamination with high colony counts of MRSA derived from an MRSA-infected patient with erythroderma was confirmed in the patient’s bed sheets, bed rail, and the floor and other surroundings of the bed. This corroborates the findings of previous reports (6, 7), that there is a high risk of MRSA contamination of the hospital environment, including of the bed sheets and floor, through scales and exudates from patients with exfoliative dermatitis. In addition, our study revealed limited MRSA isolation from the over-bed table, mobile phone, bedside table, and wash stand, showing a broader range of MRSA contamination than in previous reports. Thus, it is suggested that erythroderma patients who are infected with/carriers of MRSA can be an important source of serious and extensive MRSA contamination of the environment.
Similar to staphylococci, MRSA cells scatter in the air together with dust and skin scales when the bed is being made. Although the probability of airborne inoculation is low, it is reported as a cause of new carriers and infected patients (19). In our patient, who showed prominent production of scales, it is highly likely that these scales were scattered in the air. We also measured the MRSA cell counts scattering in the air at the time of bed sheet change in another MRSA-infected patient with psoriatic erythroderma who had prominent scales. The results showed that the cell count in the air increased to approximately 300 cfu/300 l at the time of bed sheet change, a nearly 9-fold higher level than usual, and MRSA accounted for a maximum of 70% of the cells in the air. Furthermore, within 30 min, the bacterial count returned to the baseline level (approximately 30 cfu) (data not shown). Similar to that reported for general bacterial cells floating in the waiting room of the outpatient clinic (20), MRSA cells scattering in the air during bed sheet changes are also attached mainly to floating dust particles measuring more than 5 mm (range 4–8 mm) in diameter, and most of the floating particles settle on the floor within 30 min (21). Therefore, to prevent airborne infection from MRSA-infected patients with erythroderma during treatment and nursing activities, nurses and other care staff should wear masks and caps during bed sheet changes and not enter the patient’s room for at least 30 min after a bed sheet change.
In this study, MRSA strains that were identical to those apparently eliminated by microbial substitution due to antibiotic therapy were isolated from the patient’s bed sheet after an interval of 16 days (Fig. 2). Therefore, it is presumed that the MRSA survived for a certain period in the environment around the patient’s bed, and then colonized and infected the patient again. Because it has been reported that the survival period of MRSA in the hospital room is approximately 14 days (22, 23), we believe that our estimation of the survival period of MRSA is appropriate.
The basic policy for control of nosocomial infection with MRSA is early detection of the infectious source (patient) and interruption of the pathway of contact transmission of the aetiological agent. To prevent contact transmission of infection via the hands, skin, contaminated materials, etc., it is important to carry out hygiene control of the environmental surfaces that come in direct or indirect contact with infectious agents, in addition to thorough hygiene of hands, including hand washing and disinfection after work operations. However, the status of extensive MRSA contamination of the environment determined in this study indicates that when patients with skin disease accompanied by prominent scales and exudates are infected with, or become carriers of, MRSA, complete prevention of MRSA transmission to the environment is extremely difficult. In 2003, the Society for Healthcare Epidemiology of America (SHEA) emphasized the need for active surveillance culture, and recommended implementation of surveillance culture in high-risk patients prior to their admission and skin culture in patients admitted with skin injury (24). However, at present, institutions practicing such measures are limited.
It has been pointed out that metallic copper and copper alloys may induce reduction in the risk of MRSA contamination of the environment in hospitals (3, 8, 9). We reported previously, for the first time, that when a metallic copper plate was set on the MRSA-contaminated floor around the bed of a patient with MRSA skin infection in the dermatology ward, the MRSA cell count was lower on the surface of the copper plate (14). In the present study, we found that the copper film exerted bactericidal activity, not only against S. aureus, including MRSA, but also against P. aeruginosa, and that the cell counts of the contaminant MRSA and P. aeruginosa derived from a patient with scaling dermatosis decreased on the surface of the copper film taped to the patient’s bed sheet. Exfoliative dermatitis is known to be associated with a high risk of MRSA contamination of the hospital environment (6, 7). These findings indicate that metallic copper may be useful for preventing the spread of MRSA contamination of the environment. Because bed linen is most susceptible to MRSA contamination from patients infected with MRSA (25), much is expected from the future development of linen with copper-derived bactericidal properties.
It has been speculated that the bactericidal effects of metallic copper on Escherichia coli and enterococci are exerted through the production of ROS, such as hydroxyl radicals and superoxide via the Haber-Weiss reaction and Fenton reaction between copper ions and hydrogen peroxide, or through the binding of copper ions (Cu+/Cu2+) to amino-acid side chains with cysteine or histidine, which leads to DNA damage, oxidation and degeneration of structural or functional proteins or lipids, suppression of the energy metabolic system, and osmotic stress in the bacterial cells (26, 27). In this study, the bactericidal effect against S. aureus on the copper surface was inhibited over time by metal-chelating drugs (BPSA and EDTA), hydroxyl radical removers (mannitol), superoxide remover (SOD), and hydrogen peroxide-degrading enzymes (catalase) (Fig. 6). These findings indicate that, as for E. coli (26), enterococci (27), and cryptosporidium (11), the bactericidal effect of copper is attributable to the activity of ROS. In addition, considering the finding that the bacterial component targeted by ROS is DNA in cases of enterococci (27), it is presumed that the bactericidal effect on S. aureus observed in this study may also be attributable to DNA damage of the bacterial cells. Bacteria have self-repair ability through the SOS reaction when their DNA is damaged by ultraviolet rays or quinolone antibacterial agents (28). It has been reported that E. coli cells that are lacking in the RecA gene, which controls this reaction, are susceptible to DNA damage by hydroxyl radicals produced via the Fenton reaction occurring endogenously after the administration of quinolone antibacterial agents (29). This finding provides strong support for our presumption that the target of the bactericidal activity of metallic copper is the bacterial cell DNA.
The bactericidal properties of metallic copper, which depend on the ionization of copper in the presence of water molecules, seem to be influenced by physical conditions (temperature and humidity). It is reported that metallic copper and copper alloys kill MRSA rapidly within 90 min at a temperature of 20–35ºC and humidity of 20–95%, whereas the bactericidal time is prolonged to 6 h at a temperature of 4ºC (8, 30, 31). This emphasizes the need to control the room temperature and humidity, particularly in the winter in Japan, when both parameters tend to be low, when using metallic copper or copper alloy in the indoor environments for their bactericidal properties.
A comparison of the bactericidal time of metallic copper for various bacterial species revealed that it was 10 min for P. aeruginosa, including multidrug resistant P. aeruginosa (Fig. 5), 90–180 min for S. aureus, including MRSA (Fig. 4), Legionella, and other major bacteria, including E. coli, and approximately 360 min for Methylobacterium mesophilicum (data not shown). In addition, the bactericidal time for multidrug resistant Mycobacterium tuberculosis was reported to be 5–15 days (32). This raises questions about the reasons for such differences in the bactericidal effect of metallic copper against various species. It is possible that the bactericidal time of metallic copper is proportional to the length of the generation time, considering the fact that the generation time is shortest (20 min) for P. aeruginosa, and longest (15–20 h) for M. tuberculosis, and that the longer generation time of S. aureus makes it less susceptible to ROS (33). In this study, contamination of bed linen by P. aeruginosa, an important cause of nosocomial infection, second only to MRSA, was completely inhibited by metallic copper (Table SI). Therefore, it seems promising to use metallic copper as a material to prevent P. aeruginosa contamination of the hospital environment.
This study has shown that contamination of the environment arising from an erythroderma patient with MRSA infection/carrying MRSA spread extensively to the surroundings in proportion to the frequency of contact with the patient, and that contamination by MRSA and P. aeruginosa was the most frequent. In addition, we utilized the excellent bactericidal properties of metallic copper for the bed sheets, which are not easy to disinfectant, and observed a marked decrease in the contamination rate. We conclude that metallic copper is a useful material for preventing the spread of environmental MRSA/P. aeruginosa contamination. It may be possible to effectively inhibit transmission of MRSA infection in the environment if various medical implements and materials were developed using metallic copper and used in the hospital with an accurate understanding of the sites of MRSA contamination, along with proper practice of disinfection and cleaning procedures.
The authors declare no conflict of interest.
REFERENCES
