Osamu Norisugi, Yoko Yoshihisa, Kyoko Shimizu and Tadamichi Shimizu
Department of Dermatology, Graduate School of Medicine, University of Toyama, Sugitani, Toyama, Japan
Herbal medicine is widely used worldwide and is associated with side-effects such as skin eruptions. Herbal drugs are often produced by combining multiple crude drugs, mostly of plant origin. Determining which medicinal plants are associated with the herbal drugs that induce skin eruptions can therefore be difficult. This study investigated mRNA expression of several cytokines in peripheral mononuclear cells (PBMCs) from two patients with herbal drug-induced skin eruptions; one reacted to keishi-bukuryo-gan (KBG), composed of 5 medicinal plants, and the other patient reacted to senna. PBMCs (1×106) from the 2 patients were cultured for 24 h with the supernatant from the medicinal plants from KBG or senna in various concentrations, and a reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed. A high mRNA level of interleukin (IL)-4 and IL-5 was detected in PBMCs stimulated by KBG and two of its components. Senna stimulated a high level of IL-4 and IL-5 mRNA levels in PBMCs from patient with senna-induced drug reaction. Key words: cytokine; herbal drug; keishi-bukuryo-gan; senna; drug eruption.
Accepted Mar 5, 2013; Epub ahead of print Jul 1, 2013
Acta Derm Venereol 2013; 93: XX–XX.
Tadamichi Shimizu, Department of Dermatology, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, Sugitani, 930-0194, Toyama. E-mail: shimizut@med.u-toyama.ac.jp
Herbal drugs are widely used worldwide. The general public tends to believe that these agents are safe because of their natural origin; thus, they are used frequently. However, administration of herbal drugs has been reported to be associated with diverse side-effects, such as interstitial pneumonia (1), renal failure (2), liver toxicity (3) and skin eruption (4, 5). Herbal drugs are produced by combining multiple crude drugs, mostly of plant origin, but some of animal or mineral origin (6). Determining which medicinal plants are associated with herbal drugs that induce skin eruptions is therefore often difficult. This study investigated the expression of several cytokine mRNAs in peripheral mononuclear cells (PBMCs) from patients with herbal drug-induced skin eruptions, in order to establish effective methods of diagnosing the cause of such skin eruptions.
CASE REPORTS
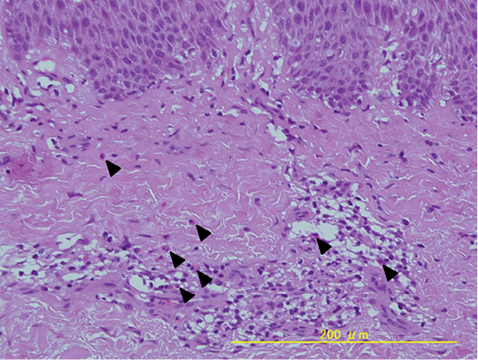
Case 1. Patient 1 was an 81-year-old woman who presented with a pruritic maculopapular rash on her entire body (Fig. 1), including her face. The patient had a 3-month history of taking the herbal drug, keishi-bukuryo-gan (KBG, also known as Gui-zhi-fu-ling-wan (in Chinese)), which was prescribed by Toyama University Hospital to treat psoriasis vulgaris. The laboratory test revealed a white blood cell count of 6,600/μl (eosinophils 18.6%). A skin punch biopsy revealed perivascular and diffuse upper dermal lymphocytic infiltrate with eosinophils (Fig. 2). The skin lesions subsided substantially within one week after stopping KBG, and the rash had completely disappeared 2 weeks later. The results of the patch test and drug lymphocyte stimulation tests (LST) for KBG were negative. However, based on the clinical findings and medical history, we suspected the patient’s eruptions to have been caused by KBG.
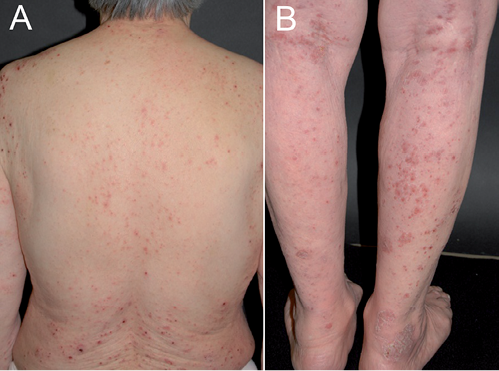
Fig. 1. Clinical appearance of the keishi-bukuryo-gan-induced skin eruption in patient 1. Erythematous maculopapular rash on the skin of her (A) back and (B) legs.
Fig. 2. Histological examination of patient 1. Perivascular and diffuse upper dermal lymphocytic infiltrate with eosinophils were observed in the lesional skin (H&E staining). Arrowheads indicate eosinophils.
Case 2. Patient 2 was a 49-year-old woman who presented with a 4-month history of pruritic erythematous plaques on her neck, trunk and extremities. The lesions had gradually enlarged during the 2 weeks prior to presentation (Fig. 3A). Her medical history showed that, over a period of several years she had taken extract of boiled senna leaf (prescribed as an over-the-counter drug) 2 or 3 times per month to treat severe constipation. The pruritic eruption appeared 1–2 days after she had started taking senna extract. Laboratory tests revealed a white blood cell count of 5,100/μl (eosinophils 14.5%). A skin punch biopsy revealed perivascular and diffuse upper dermal lymphocytic infiltrate with eosinophils. The skin lesions subsided one month after the patient stopped taking senna, and the percentage of eosinophils in white blood cells decreased to 1.1%. The results of the patch test and the drug LST for senna were negative. The patient started to take senna as a provocation test, and a similar eruption reappeared on her legs 2 days later (Fig. 3B) and the proportion of eosinophils in the white blood cells increased to 3.5%. Based on these findings, the drug eruption was suspected to have been caused by senna leaf.
Allergic symptoms, including atopic dermatitis, asthma, rhinitis and conjunctivitis were not present in either of these patients.
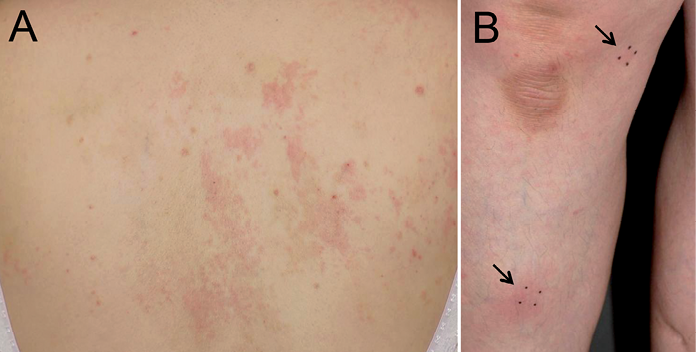
Fig. 3. Clinical appearance of the senna-induced skin eruption in patient 2. (A) Erythematous plaques appeared on the skin of the back. (B) The patient started to take senna as a provocation test, and the eruptions reappeared on her legs (arrows).
The two patients’ PBMCs were further investigated in vitro.
MATERIALS AND METHODS
Materials
KBG is composed of 5 medicinal plants (Cinnamomi cortex, Paeoniae Radix, Moutan cortex, Persicae semen, and Hoelen); these plants were obtained from the Department of Pharmacy at Toyama University Hospital. Senna leaves are commercially available. These medicinal plants were boiled and the extracts cooled to room temperature and stored at 4ºC. Furthermore, the extracts were individually suspended in RPMI 1640 (Sigma-Aldrich Co., STL, USA ) medium containing 10% foetal bovine serum (Gibco Co., Grand Island, NY, USA) and 1% streptomycin (Sigma-Aldrich Co.) and were rotated at 4oC overnight (7). The suspension was centrifuged and the supernatant filtered through a 0.45 μm-pore membrane, as described previously (7). The following materials were obtained from commercial sources: the Isogen RNA extraction kit (Nippon Gene, Tokyo, Japan); M-MLV reverse transcriptase (Gibco Co.); Taq DNA polymerase (Perkin-Elmer, Norwalk, CO, USA); and nylon membranes (Schleicher & Schuell, Keene, NH, USA).
Cell stimulation
PBMCs from patients and healthy controls (n = 3 in each experiments) were prepared from heparinized blood by Ficoll–Paque PLUS (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) density gradient centrifugation. The PBMC layer was washed 3 times with sterile PBS. PBMCs (1×106 cells/ml) were cultured in RPMI 1640 containing 10% heat-inactivated foetal bovine serum and 1% streptomycin, using 6-well plates at 37°C in a humidified atmosphere with 5% carbon dioxide. The cells were divided into 3 groups: a control group (normal healthy subjects without any treatment), a group receiving 1/100 of KBG and 5 medicinal plants or senna; and a group receiving 1/1,000 of these herbal drugs. The cell viability was evaluated by the Trypan blue (Sigma-Aldrich Co.) dye exclusion test.
Reverse transcription-PCR analysis
The total RNA was extracted from the exposed PBMCs. RNA reverse transcription was performed with M-MLV reverse transcriptase using random hexamer primers, and subsequent amplification was performed using Taq DNA polymerase. PCR was carried out for 40 cycles, with denaturation at 94ºC for 1 min, annealing from 47–50ºC for 1 min, and extension at 72ºC for 1 min using a thermal cycler (PE Applied Biosystems Gene Amp PCR System 9700, Fasmac CO. Ltd., Kanagawa, Japan ). The IL-4 primers used were: 5’-ATGGGTCTCACCTCCCAACTGCT-3’ (forward) and 5’-CGAACACTTTGAATATTTCTCTCTCAT-3’ (reverse). The IL-5 primers used were: 5’-GCTTCTGCATTTGAGTTTGCTAGCT-3’ (forward) and 5’-TGGCCGTCAATGTATTTCTTTATTAAG-3’ (reverse) (8). The RANTES primers used were: 5’-ATATTCCTCGGACACCACAC -3’ (forward) and 5’-CACTCCAGCCTGGG GAAGG -3’ (reverse). The human macrophage migration inhibitory factor (MIF) primers used were: 5’-ATGCCGATGTTCA TCGTAAAC-3’ (forward) and 5’-GGCGAAGGTGGAGTTGTTCCA-3’ (reverse). GAPDH was used as a positive control. The primers used were: 5’-ACCCAGAAGACTGTGGAT-3’ (forward) and 5’-TCGTTGAGGGCAATGCCA-3’ (reverse). After PCR, the amplified products were analysed using 2% agarose gel electrophoresis.
Statistical analysis
The values are expressed as the means ± standard deviations (SD) of the respective test or control group in cell viability. The statistically significant differences in stimulation with the tested medicinal plants were evaluated by non-parametric Mann–Whitney U test. p-values of < 0.05 were considered statistically significant.
RESULTS
Cell viability
The PBMCs were incubated with or without various concentrations of the herbal drugs or for 24 h, and cell viability was assessed. None of the treatments with KBG, comprising 5 medicinal plants or senna elicited cytoxicity in the cells from the patients and healthy controls at the tested concentrations and an incubation time of 24 h. Cell viability was > 95% (Figs S1 and S2; available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1631).
Herbal drug-stimulated cytokine expression in PBMCs
The effect of KBG on cytokine expression was examined in patient 1. The results showed high IL-4, IL-5, RANTES and MIF mRNA expression in PBMCs stimulated by KBG (at both 1/100 and 1/1,000 concentrations) and to a lesser extent by Cinnamomi cortex and Moutan cortex (Fig. 4). On the other hand, PBMCs from normal subjects exhibited no such cytokine stimulation when treated with KBG and medical plants.
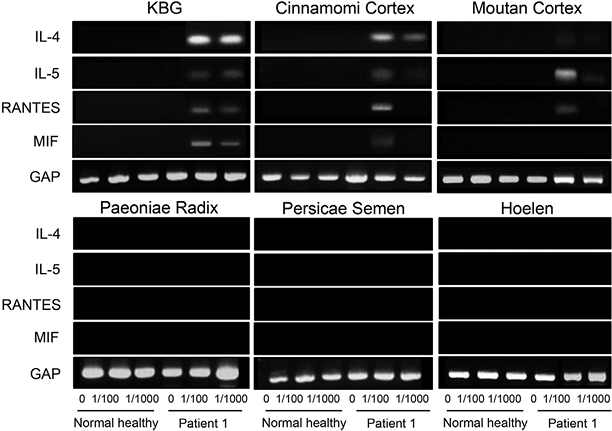
Fig. 4. Keishi-bukuryo-gan (KBG)-stimulated expression of cytokines in peripheral mononuclear cells (PBMCs) (1×106) from patient 1 with KBG-induced skin eruption. PBMCs from healthy controls were also cultured with extracts of the 5 medicinal plants from KBG at various concentrations for 24 h. Reverse transcription-PCR (RT-PCR) analysis was performed for interleukin (IL)-4, IL-5, RANTES and migration inhibitory factor (MIF). PBMCs from 3 healthy controls were used in each experiment with the same results, and the representative healthy control is shown. GAP is an internal control-
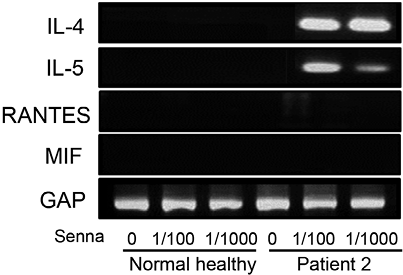
Senna stimulated a high level of IL-4 and IL-5 mRNA levels in PBMCs from patient 2 at the 1/100 and 1/1,000 concentrations (Fig. 5). However, RANTES and MIF mRNA expression was not detected and PBMCs from normal subjects exhibited no stimulation of cytokines.
Fig. 5. Senna-stimulated expression of cytokines in peripheral mononuclear cells (PBMCs). Reverse transcription-PCR (RT-PCR) analysis of interleukin (IL)-4, IL-5, RANTES and migration inhibitory factor (MIF), as described in the Methods and Fig. 4. PBMCs from 3 healthy controls were used in each experiment with the same results, and the representative healthy control is shown.
DISCUSSION
In this study, 2 patients with herbal drug eruption were examined; 1 developed skin eruptions due to Cinnamomi cortex and Moutan cortex, which comprised the medicinal plants of KBG; the second patient developed skin eruptions due to senna. Although the results of the patch test and drug LST for suspected herbal drugs were negative, both patients had eosinophilia that vanished after stopping herbal therapy. Activated eosinophil release granules containing a wide variety of mediators that can cause tissue damage and inflammation. The T-helper 2-type cytokines, IL-4, is made by these cells (9). Some of the important eosinophil chemo-attractant cytokines also include IL-5 and RANTES (10). In addition, MIF originates from multiple cellular sources, such as activated T lymphocytes, monocytes and eosinophils in allergic diseases (11). In previous studies, in vitro IL-5 production by PBMCs has been reported in patients with non-herbal drug-related skin eruptions with eosinophilia (12). A significant increase in IL-5 expression in response to drugs was noted in some patients and it was suggested that IL-5 production from sensitized mononuclear cells might be a critical mediator of drug hypersensitivity with eosinophilia, and could serve as an important diagnostic marker (12).
This study showed, in patient 1, that 2 of the 5 components of KBG, Cinnamomi cortex and Moutan cortex, were the cause of the skin eruptions. Thus, IL-4 and IL-5 mRNA were detected after stimulation of PBMCs with KBG, Cinnamomi cortex and Moutan cortex, even at the lowest concentration. Simulatory, senna stimulated a high level of IL-4 and IL-5 mRNA levels in patient 2, but RANTES and MIF mRNA were not detected in this case.
KBG has been used clinically to treat various diseases, including skin diseases (13). It has been reported that KBG improves conjunctival microcirculation in patients with cerebrospinal vascular diseases (14), thus suggesting that it may have beneficial effects on haematological parameters, such as blood viscosity and red blood cell deformability (15–17). KBG is now one of the most frequently used medicines in Japan and KBG-induced drug eruptions have been reported in the Japanese scientific literature. In this study, we found that Cinnamomi cortex and Moutan cortex cause KGB-induced drug eruption. Determining the individual roles of medical plants in drug eruption is valuable, as these plants are included in many herbal drugs other than KBG, which patients should avoid using.
Senna is a major laxative herbal drug derived from the leaves/pods of Cassia acutifolia and C. angustifolia (India or Tinnevelly senna). It is generally believed to be a safe agent because of its natural origin. Therefore, patients tend to use it frequently and persistently as self-medication for constipation. The known side-effects of senna are abdominal pain and electrolyte imbalance. Pseudomelanosis coli proliferation (18) and potential neoplastic changes in the gut (8) have also been reported. There have been several reports of other senna drug eruptions in dermatology journals (19, 20).
Both of our patients showed negative patch test and negative LST results. Nevertheless, herbal drug-induced eruptions are type IV hypersensitivity reactions and patients with fixed-type drug eruptions or severe forms of drug reactions caused by herbal drugs often show positive skin patch tests (4, 21). The provocation test sometimes helps in the diagnosis of herbal drug-induced skin eruptions when the patch test is negative (22, 23). In addition, some crude herbal drugs may give false-negative LST results. For example, Mao (an ephedra herb, E. herba) had a low lymphocyte stimulation index (< 90%) even in healthy volunteers (23). Therefore, the determination of T-helper 2-type cytokine expression, such as the levels of IL-4 and IL-5 in PBMC cultures, may be helpful in confirming the diagnosis of herbal drug-induced skin eruptions in patients who have had eosinophilia, even when the patients have a negative patch test and negative LST. In addition to medical herbal drugs for systemic usage, herbal foot baths, herbal pillows, herbal lotions, and herbal shampoos are common everyday items, which may be overlooked as a cause of skin eruptions in some patients (25).
In conclusion, these findings indicate that the measurement of medicinal plant-induced IL-4 and IL-5 mRNA in PBMCs may be a useful in vitro diagnostic tool for identifying the cause of herbal drug-induced skin eruptions.
Acknowledgements
This research was supported by a Grant-in-Aid for Scientific Research (number 20591337) from the Japan Society for the Promotion of Science.
REFERENCES
