Maria Kitchen1, Hansgeorg Müller1, Alexandra Zobl2, Andrea Windisch2, Nikolaus Romani1 and Hartwig Huemer2
Departments of 1Dermatology and Venereology, and 2Hygiene, Microbiology and Social Medicine, Division of Virology, Innsbruck Medical University, Innsbruck, Austria
A variety of animals host parapoxviruses. Orf virus is prevalent in sheep and goats in the Tyrol region of Austria and Northern Italy. Zoonotic infections in humans mostly occur after occupational exposure. We report here a case of a hunter with a typical Orf lesion (contagious ecthyma) on the finger, with no history of direct contact with domestic animals. Three weeks previously he had been hunting chamois (Rupicapra rupicapra) and cut his finger while handling a carcass. Parapoxvirus infection was confirmed by electron microscopy and PCR, and the species was identified by DNA sequencing. The sequence was highly homologous with prevalent sheep Orf virus and rather distant from parapoxviruses found in red deer in Northern Italy. As this case indicated that the infection was acquired via game, we performed spot testing in the suspected area and detected several seropositive animals. This is a strong indication that Orf virus has been introduced into chamois in Western Austria. This probably occurred via roaming domestic sheep sharing the high alpine areas during the summer months. Key words: parapoxvirus; orf virus; zoonosis; game; chamois; hunting; skin infection.
Accepted Mar 26, 2013; Epub ahead of print Aug 27, 2013
Acta Derm Venereol 2013; 93: XX–XX.
Maria Kitchen, Department of Dermatology and Venereology, Innsbruck Medical University, Anichstrasse 35, A-6020 Innsbruck, Austria. E-mail: maria.kitchen@uki.at
Orf virus is a poxvirus, occurring worldwide, which causes contagious ecthyma in sheep and goats. The virus can remain infective for many weeks, especially when embedded in proteinaceous crust material, thus enabling widespread infection in animal herds. As parapoxviruses do not lead to persistent immunity animals can be infected repeatedly (for review see (1)). Orf is also a zoonotic disease that can spread to humans after contact with infected animals or contaminated fomite. In humans it mostly manifests as proliferative and ulcerative skin lesions after occupational exposure on the hands, but multiple lesions can occur (2). The lesions usually heal without scarring.
Case report
A 51-year-old man was admitted to our dermatology unit with a skin lesion on the middle finger of the left hand. He is a recreational hunter and had been hunting a chamois (Rupicapra rupicapra) 23 days earlier, when he sustained a cut on the finger while field-dressing the carcass. The injury was unremarkable, but did not heal, and during the following weeks a violaceous nodular lesion developed, which did not improve despite antiseptic treatment. On another hunting excursion (10 days after the first) the patient had also handled the carcass of an Alpine marmot (Marmota), but had not sustained any new injuries.
At the time of presentation to our hospital the patient had 2 nodular skin lesions around the proximal interphalangeal joint of the left middle finger (Fig. 1), as well as some surrounding erythema and swelling. Painful lymphangitis and enlarged axillary glands prompted the patient to consult our emergency department. Otherwise he was in good general health without constitutional symptoms. The physical examination and vital parameters were unremarkable. Routine blood tests, including serology for tularemia, were normal. A skin biopsy was taken for histology and microbiology, and scrapings were examined under the electron microscope (see Results).
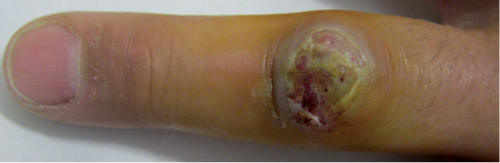
Fig. 1. Orf virus-induced papulonodular skin lesion. Nodular skin lesions over the proximal interphalangeal joint of the third digit of the left hand of the patient 23 days after exposure.
While the lymphangitis responded to intravenous cefazolin followed by oral cefalexin with rapid resolution (1 g every 8 h for a total of 9 days), the nodular skin lesions on the finger showed very slow improvement with topical antiseptic measures (povidone-iodine dressing initially (Inadine®, Systagenix, Gargrave, UK), later dry sterile dressing). The finger was immobilized. No specific antiviral treatment was given. The lesions finally healed without scarring within 8 weeks.
MaterialS and Methods
Electron microscopy and molecular diagnostics
Scrapings of the skin lesion were negatively stained with 2% phospho-tungstic acid and viewed on a Philips EM400 electron microscope (FEI Company, Eindhoven, The Netherlands) at an operating voltage of 80 kV (3). PCR orthopoxvirus und parapoxvirus specific primers were used as described previously (4, 5). The amplified DNA sequences were analysed by automated sequencing using an Applied Biosystems ABI3140 automated sequencer. The obtained sequences were aligned with homologous sequences downloaded from GenBank (www.ncbi.nlm.nih.gov/genbank/) using the programs Proseq3 (http://dps.plants.ox.ac.uk/sequencing/proseq.htm) and ClustalX (http://www.clustal.org/). The homology was evaluated by the neighbour-joining algorithm with bootstrap analysis using 100 pseudoreplicates. Table SI (available from http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1643) lists the virus strain sequences included for comparison.
Cell culture and serology testing
Several cell types were used for virus culture, i.e. MDCK (canine kidney), LLC-PK1 (porcine kidney), Vero (monkey kidney), A569 (lung epithelial), and A431 (keratinocyte), grown on RPMI1640 medium supplemented with 10% foetal calf serum (FCS), glutamine and penicillin/streptomycin (all reagents from SIGMA-Aldrich, St. Louis, MO, USA). Serology testing for anti-parapoxvirus antibodies was performed using indirect immunofluorescence following standard procedures using Vero cells infected with Orf virus strain BO15 (kindly provided by M. Büttner, Oberschleissheim, Germany). In brief, 12-well diagnostic slides (Thermo Scientific, Waltham, Massachussetts, USA) were coated with cells infected 4 days earlier and then air dried. Thereafter the slides were fixed with a mixture of ice-cold acetone and methanol, sealed under vacuum and stored in the freezer until further use. Different dilutions of human or animal serum were performed in phosphate buffered saline (PBS) containing 10% FCS and the wells incubated for 1 h in a wet chamber. After several washings in PBS a secondary antibody was applied at the dilutions indicated by the supplier of the reagent. For detection of human serum fluorescein (FITC)-conjugated rabbit immunoglobulins to human IgG (DAKO, Glostrup, Denmark) was used. Antisera from chamois were tested with a mixture of FITC-labelled rabbit anti-goat IgG heavy and light chain (from KPL, Gaithersburg, MD, USA) and FITC-labelled rabbit anti-sheep IgG antibody (DAKO). The second antibody was applied together with a 1:1000 dilution of Evans blue 1% (w/v) stock solution as a fluorescent counter-stain. After a further 1 h incubation the slides were washed again with PBS, mounted with PBS-buffered 80% glycerol under glass-cover slides and immediately visualized with a Zeiss fluorescent microscope. A clear fluorescence at a dilution of 1:40 was considered positive. Blood samples from 16 recently shot chamois were kindly provided by the local hunting community of the Sellrain valley in the North Tyrol region, where the suspect case originated.
ResultS
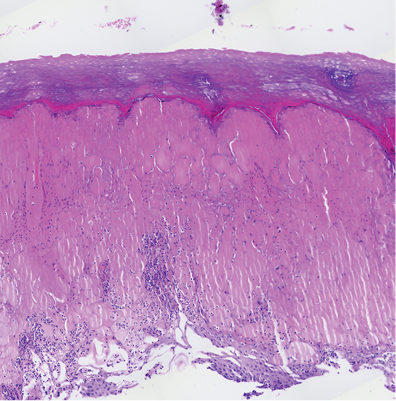
The haematoxylin and eosin (H&E) stain of the lesional biopsy is shown in Fig. 2. Microbiological stains (Gram, periodic acid Schiff (PAS), methylene blue) were negative.
Fig. 2. Histology of a lesional biopsy (haematoxylin and eosin; H&E). Acral skin with compact hyperkeratosis overlying intraepidermal vacuolar changes with massive ballooning, haemorrhagic bullae, and pustules; mixed inflammatory cell infiltrate with a prominent neutrophilic component (H&E, × 200).
Both electron microscopy and PCR indicated a parapoxvirus infection. Electron microscopy disclosed the typical oval-shaped parapoxvirus particles, mainly in the M form (Fig. 3).
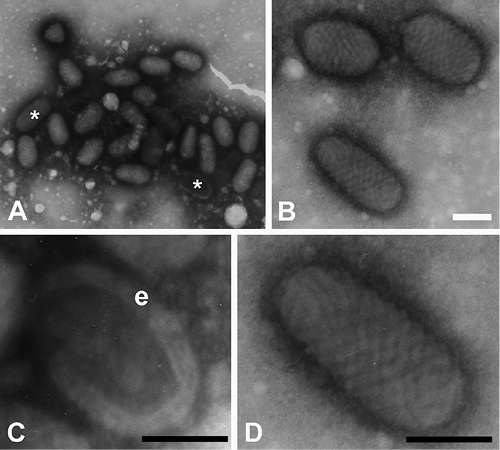
Fig. 3. Electron micrographs of parapoxvirus particles in scrapings from skin lesion. Electronmicroscopy (negative stain). (A) Virus particles were observed in large groups. (B–D) Higher magnifications of parapox viruses. They occur mainly in the M (mulberry) form (without envelope), but also in the C (capsule) form (* in A, and panel C). The thick viral envelope (e, in panel C) can be clearly recognized. Note also the ovoid shape and the typical spiralled surface pattern. Scale bars indicate 100 nm, i.e. the approximate width of the virus.
DNA sequencing of the fragments amplified by the parapoxvirus specific primers revealed an Orf virus sequence highly homologous with sheep strains (Fig. S1; available from http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1643). The sequence is referred to as ORFV-AUT/2012/Sellrain and deposited in GenBank accession # HE996965 (www.ncbi.nlm.nih.gov/genbank/).
The primary isolate grew slowly on MDCK and LLC-PK1 cells, but not on Vero, A569 or A431 cell lines, whereas the laboratory-adapted strain (BO15) was also able to replicate in Vero cells (not shown).
Serological testing of the 16 chamois samples revealed 3 animals that were clearly seropositive (Fig. S2; available from http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1643). The serum of the hunter had a high antibody titre in indirect fluorescence assay exceeding 1:1000 in the second serum sample taken 5 weeks after the infection (not shown).
DISCUSSION
Parapoxvirus infection was rapidly confirmed by electron microscopy. Virus particles occurred mainly in the M-(mulberry) form (without envelope). Both M and C-(capsule) forms are commonly observed in negatively-stained specimen from tissue culture or, as in this report, in patient isolates. The C-form seems to be a degenerate form of the M-form, often associated with dry scabs (6).
The homology with sheep strains was remarkable, since there is no evidence of direct contact between the patient and sheep or sheep material. It is most likely that the infection was contracted while butchering the chamois carcass 3 weeks before presentation to our hospital, on which occasion a minor injury occurred on the finger. However, we cannot rule out that the origin of the Orf virus infection was an unknown environmental source, such as grass or leaves contaminated by infected sheep, or even contact with a marmot 10 days after the original contact with the chamois, although no additional skin wounds were acquired on this occasion. The clinical course of the infection was unremarkable, the lesions healed within 8 weeks. Since the patient was not immuno-compromised and the lesions did not increase after presentation to our centre, we did not consider treatment with antiviral agents, such as topical cidofovir or imiquimod.
There are very few data available on parapoxvirus infection in game in middle Europe, and seroprevalence data are lacking. There are only 2 isolates from chamois listed in GenBank at present. However, from the inclusion of 3 chamois-derived isolates (of undisclosed origin and unpublished sequence) in an Italian publication, it can be assumed that there are/were such infections in Italy (7). A more recent study by the same investigators detected isolates in red deer in Northern Italy, which appear to be more closely related to the parapoxvirus found in red deer in New Zealand (PVNZ) than to the classical Orf virus (8). The reason for this clustering in red deer in Italy may be due to hunters winter-feeding deer at accessible lower altitude areas. In contrast, solitary game, such as chamois, live high up in the mountains and do not usually gather for winter feeding. However, during the summer months these animals have contact with free-roaming domestic sheep on high mountain pastures. It is therefore possible that chamois may sporadically be infected by sheep strains of Orf virus.
In the Eastern USA parapoxvirus infection was recently described in 2 deer hunters. Their infection was caused by a novel strain of virus clustering with pseudocowpox virus (PCPV), which could indicate a spill-over from domestic cattle to deer (9). In contrast, our case demonstrates an Orf virus sequence that was probably transferred from sheep to chamois.
In farm animals, Orf virus infections are diagnosed primarily on clinical grounds and there are no epidemiological data for our region. In humans, Orf virus infections occur in people handling sheep and goat. They are not notifiable and could be underdiagnosed, since there are rarely constitutional symptoms, and skin lesions eventually heal spontaneously.
If Orf viruses are spreading to species other than sheep and goat, Orf virus exposure might occur more frequently in persons not considered at risk for this infection.
Acknowledgements
The authors appreciate the strong support they received from the hunters’ community of the Sellrain Valley, Tyrol, Austria, providing chamois blood/tissue material. The authors would also like to thank Dr Walter Glawischnig, Austrian Agency for Health and Food Safety (AGES), and Professor Matthias Büttner, Bavarian Health and Food Safety Authority (LGL), Oberschleissheim, Germany, for providing control sera and virus material.
References
