Hee-Chul Chung1, Bo-Kyung Kim1, Hannah Hong1, Hye-Young Wang2, Yeun Kim3, Hyeyoung Lee3, Minseob Eom4 and Eung Ho Choi1*
Departments of 1Dermatology and 4Pathology, Yonsei University Wonju College of Medicine, 20 Ilsan-ro, Wonju, 2M&D, Inc., Wonju Eco Environmental Technology Center and 3Department of Biomedical Laboratory Science, College of Health Sciences, Yonsei University, Wonju, Korea. *E-mail: choieh@yonsei.ac.kr
Accepted Jun 15, 2015; Epub ahead of print Jun 18, 2015
Cutaneous tuberculosis (TB) is traditionally diagnosed via histopathological findings of granuloma with caseous necrosis and positive acid-fast bacilli (AFB) staining or culture. Tuberculids, such as erythema induratum (EI), which are associated with TB, may demonstrate atypical characteristics of TB, including negative TB culture or staining and, histologically, non-caseating, non-granulomatous features. EI and erythema nodosum are difficult to distinguish clinically and histopathologically; therefore, making a correct diagnosis is difficult (1). The gold standard for a definite diagnosis of TB is detection of Mycobaterium tuberculosis on culture; however, this method is time-consuming, resulting in a delayed diagnosis (2).
The interferon-γ (IFN-γ) release assay estimates the ability to produce IFN-γ, using Mycobacterium antigens to stimulate T cells. This assay has a low false-positive rate, regardless of BCG vaccination, and high specificity, and can therefore be used to diagnose both latent and active TB (3). The aim of our report was to examine the diagnostic usefulness of the interferon-γ release assay and the reverse blot hybridization assay (REBA) in 7 patients suspicious of having cutaneous TB not identified by conventional methods including TB-PCR.
CASE REPORTS
The IFN-γ release assay (QuantiFERON®-TB Gold, Cellestis Ltd, Carnegie, Victoria, Australia) and REBA (REBA Myco-ID®, M&D Inc, Wonju, Korea) were used in this study. REBA is a method of hybridizing the PCR products of DNA extracted from Mycobacterium-infected specimens on a prepared DNA template of Mycobacteria, including M. tuberculosis. REBA provides a result within 48 h with high sensitivity and specificity (4–8). From July 2007 to July 2014, 7 patients with various clinical features on their lower extremities and neck were enrolled in this study (Fig. 1). The following data were collected: history of TB, family history of TB, diagnostic studies including TB culture, IFN-γ release assay by TB, TB-PCR, REBA for Mycobacterium, chest radiography, etc. (Table SI1). There were no specific findings in blood and urine analysis in all patients. All patients showed histopathological findings suspicious of cutaneous TB including tuberculid, but negative TB-PCR results in paraffin sections of skin specimens. All patients presented a positive M. tuberculosis complex in REBA for Mycobacteria and a positive IFN-γ release assay for TB. The individual test results and treatments are demonstrated in Table SI1.
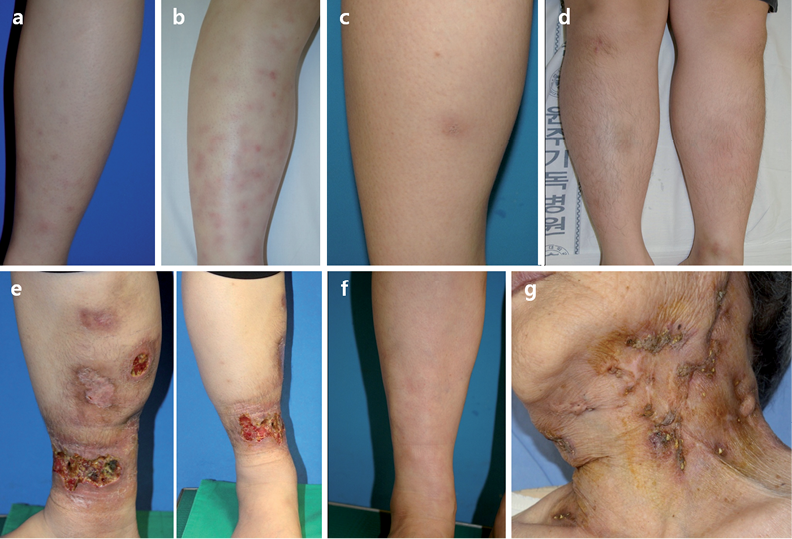
Fig. 1. Five patients (cases 1–5; a–e) presenting single or multiple erythematous-to-brownish subcutaneous nodules, multiple severe ulcerative plaques and scars, and lobular panniculitis with vasculitis in histopathology, were diagnosed as erythema induratum. Case 6 (f) presenting similar clinical features, but septal panniculitis on histopathology, was diagnosed with erythema nodosum. Case 7 (g) showed multiple brown ulcerative lesions and traces of abscess on the neck and, histologically, irregular epidermal hyperplasia with focal giant cell infiltrates. Case 7 also had a history of TB infection, and was diagnosed with scrofuloderma.
Four patients who were not improved by conventional treatments for EI (9), including anti-inflammatory drugs or oral corticosteroids, were cleared by treatment with anti-TB medication composed of isoniazid, rifampin, ethambutol and pyrazinamide for 6 months (Table SI1).
DISCUSSION
The IFN-γ release assay is a rapid and accurate blood test, avoiding the need for biopsy, and allowing detection of the host immune response to Mycobacterium antigens in vitro (10). It has high sensitivity (89%) and specificity (98%), because it estimates the immune response against TB proteins that are not included in the BCG vaccination (3). Prospective studies have shown that the IFN-γ release assay has a higher predictive value in the diagnosis of latent TB infection than the conventional tuberculin skin test (11), and an overall 92–98% agreement with the results of conventional isolation and identification methods (4).
REBA is a probe-based method, in which multiple oligo-nucleotide probes are immobilized on nitrocellulose strips and hybridized with biotin-labeled PCR products (12). This assay can differentiate 18 mycobacterial species, using specific probes for the rpoB gene region (Fig. S11). The data were interpreted using the REBA Myco-ID data sheet provided by the manufacturer (6, 13).
For the definitive diagnosis of cutaneous TB, smear microscopy and/or mycobacterial culture have been used, but smear microscopy has poor sensitivity due to the low number of bacilli. The positivity of mycobacterial cultures of fine-needle aspiration cytology samples is between 35% and 65% due to the paucibacillary nature of the specimens (6). AFB stain has low sensitivity, and culture takes 6–8 weeks, resulting in delays in treatment, whereas REBA provides a rapid result, within 48 h. PCR and REBA work on the same basic principle, but REBA may increase sensitivity by amplifying PCR results using the reverse blot hybridization assay. A positive result could be obtained by REBA, despite being negative in PCR, because the sensitivity of REBA is 95–100%, which is greater than the sensitivity of real-time PCR (6–8).
Six of our patients were diagnostically challenging, since the causative M. tuberculosis was not detected on staining or on culture from skin samples. However, positive results were obtained with the REBA and 4 of these patients responded to TB medication. Therefore, REBA could be useful for the diagnosis of cutaneous TB. Several reports have shown that some nucleic acid amplification methods are very sensitive and specific for bacterial culture or clinical specimens, but are not highly sensitive for formalin-fixed paraffin-embedded tissues. This may be due to DNA degradation during the fixation process (6).
In conclusion, the IFN-γ assay and REBA could be fast and reliable tools for diagnosis of cutaneous TB, especially in suspicious cases that have not been diagnosed by conventional methods.
ACKNOWLEDGEMENT
Funding sources: This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2687).
The authors declare no conflicts of interest.

1http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-2182
REFERENCES
