Lu Zhou, Hong-Sheng Wang*, Su-Ying Feng* and Qiu-Ling Wang
Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, China
*These authors contributed equally to this article.
Mycobacterium intracellulare-caused pulmonary infections have mostly been reported in immunocompromised hosts, while cutaneous M. intracellulare infections are rare. We describe here an immunocompetent patient with cutaneous lesions due to M. intracellulare, which was diagnosed by acid-fast staining, in vitro culture, histopathology, and PCR-restriction fragment length polymorphism analysis and gene sequencing of heat-shock protein (hsp) 65 and 16S rDNA genes. In vitro susceptibility testing was also carried out and the patient was successfully treated with clarithromycin, rifampicin, and ethambutol. Key words: skin disease; infectious; mycobacteria, intracellulare; heat-shock protein 65; RNA, Ribosomal, 16S.
Accepted Jan 16, 2013; Epub ahead of print Mar 26, 2013
Acta Derm Venereol 2013; 93: XX–XX.
Hong-Sheng Wang, Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, 12 Jiangwangmiao Road, Nanjing 210042, China. E-mail: whs33@vip.sina.com; fengsuying2010@yahoo.com.cn
Mycobacterium intracellulare-caused pulmonary infections have mostly been reported in immunocompromised hosts. This organism belongs to the group of non-tuberculosis mycobacteria (NTM). Cutaneous M. intracellulare infections are rarely reported. We describe here a case of cutaneous infection due to M. intracellulare in an immunocompetent person.
CASE REPORT
A 42-year-old male farmer presented with asymptomatic reddish papules and nodules on his back and face. Eighteen years previously, skin lesions first appeared as reddish papules on the patient’s right face. These lesions gradually increased in size and number and then developed into erythematous infiltrated plaques and nodules with slight scales, atrophy, and alopecia, finally extending to the whole right side of the patient’s face, ear, and neck. Similar lesions appeared on his back approximately 5 years ago, but these only developed reddish papules with partial atrophy. No clinical manifestations of ulceration, vesicle/bullae, or sensory dysfunction were observed. The patient had not undergone previous intervening medical treatment before he was referred to our outpatient department for evaluation of his skin lesions. He did not recall any potential inducement of the lesions or previously receiving immunosuppressant therapy.
Dermatological examination revealed a large area of erythematous infiltrated plaques and nodules with slight scales, sporadic atrophy, scars, and secondary alopecia, all of which were located on the right side of the face and neck (Fig. 1a, b). A single well-circumscribed reddish plaque on the patient’s back also presented with some scales, atrophy and scars (Fig. 1c). Neither cervical nor axillary lymph nodes were palpable. Routine laboratory examinations showed that the patient was generally in good health. The result of the purified protein derivative of tuberculin test was also negative. Systemic examinations of chest X-ray, type-B ultrasonic and computed tomography (CT) scan revealed no abnormal lesions in the internal organs. A skin biopsy of the lesion on his back showed a normal epidermis with nodular and non-caseating granulomas infiltrated by lymphocytes, histiocytes, multinucleate giant cells and foamy cells, as well as panniculitis of lymphocyte infiltration around fat lobules, resulting in the histopathological diagnosis of infectious granuloma (Fig. 1g, h). Ziehl-Neelsen staining revealed that acid-fast bacilli (AFB) were suspiciously positive in the granuloma tissue (Fig. 1i). Fungal, mycobacterial and other bacterial cultures showed that mycobacterial culture was positive, whereas the other cultures were all negative. The moderate growth of smooth colonies was noted after 20 days of incubation at 32ºC and 37ºC on Löwenstein–Jensen (L-J) medium. Ziehl-Neelsen staining confirmed that the cultured organisms were AFB. The isolated strain could not grow on L-J medium at 25ºC or 45ºC, even after 3 months of inoculation. All of the above cultures had been repeated at least 3 times, respectively, resulting of the same outcomes. Pigmentation production testing showed that the isolated AFB was non-chromogenic and belonged to Runyon III. In vitro susceptibility testing using the microtitre plate method in accordance with CLSI guidelines showed that the isolated organism was sensitive to clarithromycin, rifampicin and ethambutol (1). Further identification of the isolated mycobacteria by PCR-RFLP and gene sequencing of the hsp65 and 16S rDNA genes was carried out (2, 3). A 439-bp DNA fragment that encoded mycobacterial 65-kDa heat shock protein was amplified by PCR from the strain, and digestion of the PCR product yielded 3 fragments of 235, 120 and 100 bp with BstEII and 3 fragments of 145, 130 and 60 bp with HaeIII (Fig. 2). We used a public web server (available from: http://app.chuv.ch/prasite/index.html) that utilizes pattern recognition to identify the isolate strains from their RFLP patterns. The RFLP pattern of the isolated strain was identical to that of M. intracellulare. The sequences were analysed with the BLAST V2.0 software available from http://www.ncbi.nlm.nih.gov/BLAST/. Likewise, the sequence analysis of the hsp65 and 16S rDNA genes revealed 99% and 100% homology, respectively, with M. intracellulare strain ATCC 13950 (GenBank accession number AF126035.1 and GQ153276.1). Based on previous reports of M. intracellulare infection (4–9), we performed additional laboratory tests and systemic examinations. The cell-mediated immunity level in the peripheral blood cells was detected by flow cytometry, and the CD4+ and CD8+ T-cell counts were normal. The result of HIV antibody detection testing was negative. An X-ray of the chest again yielded normal results. As indicated by the drug susceptibility analysis, we administered an oral regimen of clarithromycin (1.0 g/day), rifampicin (450 mg/day) and ethambutol (750 mg/day). After 3 months of therapy, the skin lesions greatly improved and remarkable regression of the lesional papules and nodules was observed. The patient ceased systemic therapy after one year with complete cure (Fig. 1d, e, f). The patient has been followed up for more than 2 years with no sign of relapse.
Fig. 1. Clinical manifestations and histopathological findings. Mass of erythema, plaques and nodules on (a) the face and (b) the neck and (c) a single reddish plaque with scales on the back, were observed at the initial visit. (d–f) After 3 months of treatment with systemic rifampicin, ethambutol and clarithromycin, the skin lesions gradually healed with hyperpigmentation and scar formation. (g, h) Lesional skin biopsy on the back showed granulomatous of inflammatory cell infiltrates, including lymphocytes, histiocytes, multinucleate giant cells and foamy cells in the whole dermis and panniculitis of lymphocytes infiltration around fat lobules (H&E × 200). (i) Ziehl-Neelsen staining for acid-fast bacilli was positive in granuloma.

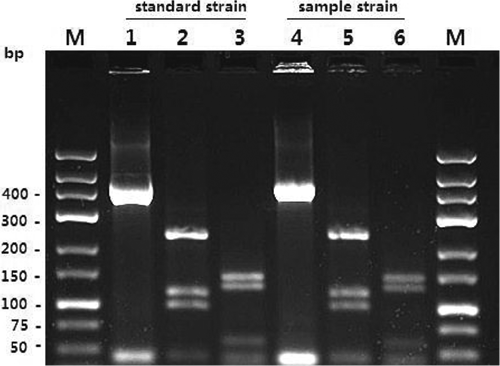
Fig. 2. Electrophoretic profiles of the PCR products after BstEII and HaeIII digestion. The standard and sample origins of the M. intracellulare isolates (lanes 1–3 and 4–6) are indicated. M: marker; lanes 1 and 4: 439 bp (hsp65); lanes 2 and 5: after BstEII digestion; and lanes 3 and 6: after HaeIII digestion.
DISCUSSION
M. intracellulare is one of the most common pathogens involved in the opportunistic infection of patients with AIDS (10). However, cutaneous infections caused by M. intracellulare have rarely been reported, either in immunocompetent (11–13) or immunosuppressed (8) patients (Table Ι). Our patient tested negative for HIV antibodies and had normal humoral and cellular immunity status. He recalled no history of any kind of skin injury, and the probable cause of our case remains unknown. In fact, we excluded systemic involvement prior to diagnosis of cutaneous infection. Systemic checks had been operated through chest X-ray, type-B ultrasonic and CT scan of chest and abdomen without any visible abnormality. Thus, we considered the lesion on the face could be first triggered by minor wounds, directly contacted with infectious water, soil, etc., and then sequentially, the pathogens may have inoculated directly on his back after years without any chemotherapy. The clinical manifestations of cutaneous infection by M. intracellulare are unusual and may present variously as abscesses, erythematous plaques with yellow crusted bases, nodules due to abscess formation or panniculitis, ulcerations, sinus tracts, and folliculitis (6, 9, 14). In our case, the lesions mainly showed erythematous infiltrated plaques and nodules with scales, atrophy and scars, as well as secondary alopecia. The non-specific appearance of the lesions did not allow easy and definite diagnosis at first sight. Some differential diagnoses for similar clinical presentations should also be considered, such as fungal infections, cutaneous tuberculosis, leprosy, and inflammatory or tumour disease.
Table I. Clinical characteristics of previously reported patients with cutaneous infections due to M. intracellulare
|
Case |
Reference |
Age, |
Affected skin |
Immune status |
Antibiotics |
Disease duration |
Outcome |
|
1 |
Cox & Strausbaugh (11) |
53/F |
Disseminated |
Immunocompetent |
Isoniazid, ethambutol, hydrochloride, rifampicin |
11 years |
Died |
|
2 |
Sachs et al. (12) |
80/F |
Right anterior tibia |
Immunocompetent |
Ethambutol, ansamycin |
16 months |
Cured |
|
3 |
Yamada et al. (13) |
12/M |
Lumbar region and buttock |
Immunocompetent |
Clarithromycin |
5 months |
Cured |
|
4 |
Saruwatari et al. (8) |
22/M |
Both lower legs |
Immunocompromised |
Clarithromycin |
10 weeks |
Cured |
The diagnosis of NTM infection requires a series of laboratory tests to identify and exclude suspicious diagnoses that may later affect treatment. Identification of NTM by conventional examinations, including acid-fast staining, in vitro culture, and histopathology, is difficult (4, 15). Initially, we performed traditional acid-fast staining (Ziehl-Neelsen staining), as well as cultures on L-J medium and drug susceptibility analysis. For the strains from colonies on the L-J medium, novel technologies, such as PCR, PCR-RFLP analysis, genetic sequencing analysis, commercial DNA probes and high-performance liquid chromatography (HPLC) have emerged as able to identify NTM at the species level (4, 16, 17). Amplifications of the hsp65, and 16S rDNA genes could help classify the sample strains between genotypes of M. intracellulare; hsp65 analysis has a greater ability to resolve M. avium-intracellulare complex (MAC) strains (16). Therefore, in this study, PCR was used to amplify specific fragments of the mycobacterial hsp65 and 16S rDNA genes, and these sequences were subsequently compared using the BLAST program against the GenBank database to identify the specific NTM as M. intracellulare. PCR-based techniques for the direct detection of mycobacteria DNA in skin samples may facilitate the diagnosis of cutaneous mycobacterial infections (18–21). However, the positivity of PCR in the detection of cutaneous mycobacterial infections is still controversial.
The recommended treatment for MAC involves a combination of ethambutol, clarithromycin/azithromycin, and rifampicin/rifabutin for 3–6 months or longer (7). For immunocompetent patients with extrapulmonary disease, continuous chemotherapy for 18–24 month is recommended (9). Clarithromycin or azithromycin must be administered in combination with other agents, such as ethambutol, to prevent the emergence of macrolide resistance (9).
In conclusion, M. intracellulare infection is increasingly reported as a pathogen. The diagnosis of this rare cutaneous infection should be considered, not only in immunocompromised patients, but also in patients with normal immunity. Mycobacterial species identification and drug susceptibility analysis should be performed prior to therapy and prognosis.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Natural Science Foundation of China, 2010 (30972651); and the fund for Key Clinical Program of the Ministry of Health of China, 2010 (2010-2012-125).
REFERENCES
