Yumi Kambayashi, Taku Fujimura and Setsuya Aiba
Department of Dermatology, Tohoku University Graduate School of Medicine, Sendai, Japan
An imbalance of immunosuppressive and immunomodulatory cells plays an important role in inhibiting the anti-tumour immune response in a tumour-bearing host. Among such cells, regulatory T cells (Tregs), together with immunosuppressive macrophages, such as CD163+ M2 macrophages, play roles in maintaining the tumour microenvironment. In contrast, interleukin-27 (IL-27) induces STAT1 and STAT3 activation, thus resulting in the enhancement of naive CD4 T-cell proliferation, the promotion of early Th1 differentiation, and the induction of the anti-tumour immune response. The purpose of this study was to investigate the involvement of immunosuppressive cells, such as Tregs and CD163+ macrophages, as well as immunomodulatory cells (i.e. IL-27-producing cells) in keratoacanthoma (KA) and invasive squamous cell carcinoma (SCC). We also examined the presence of CD3+ Foxp3+ Tregs cells in lesional skin from 10 patients with KA and 18 patients with SCC. Increased numbers of CD3+ Foxp3+ Tregs were observed in SCC compared with KA. In parallel with Tregs, higher numbers of CD163+ macrophages and MMP-9+ cells were detected only in SCC. In contrast, IL-27-producing cells were increased only in KA. In addition, the expression of pSTAT1 on tumour cells was observed only in KA. These findings suggest that the induction of immunosuppressive and immunomodulatory cells differs between KA and SCC. Key words: keratoacanthoma; squamous cell carcinoma; regulatory T cells; Foxp3; CD163+ M2 macrophages; MMP-9; IL-27; pSTAT1.
Accepted Jan 17, 2013; Epub ahead of print Apr 10, 2013
Acta Derm Venereol 2013; 93: XX–XX.
Taku Fujimura, Department of Dermatology, Tohoku University Graduate School of Medicine, Seiryo-machi 1-1, Aoba-ku, Sendai, 980-8574, Japan. E-mail: tfujimura1@mac.com
Keratoacanthoma (KA) is a benign neoplasm that regresses spontaneously after 3–6 months and shares features with squamous cell carcinoma (SCC). The histo- pathological diagnosis of KA is based on its architecture as well as cytological features (1). Clausen et al. (2, 3) reported significantly different regions of genomic aberrations for KA and SCC, based on chromosomal comparative genomic hybridization (CGH). Moreover, Li et al. (4) reported chromosomal instability during the whole life cycle of KA. Although typical cases of KA can be distinguished from SCC (1), sometimes it is histopathologically difficult to discriminate KA from SCC. In addition, an increased incidence of KA is seen among immunosuppressed patients (5), which suggests that the immunological environment contributes to the development of KA. Therefore, in this study, we employed immunohistochemical staining in order to find discriminating markers for KA and well- or moderately-differentiated SCC.
Immunological tolerance of self-antigens is essential for the prevention of autologous reactions and autoimmune diseases. In the peripheral organs, tolerance is reinforced by a variety of mechanisms, including a population of regulatory T cells (Tregs) that actively suppress the function of autoreactive T cells. These Tregs are identified by the expression of Foxp3, CD4 and the interleukin (IL)-2Rα chain (CD25). Foxp3, a member of the forkhead family of transcription factors, is both necessary and sufficient for the development and function of those CD4+CD25+ Tregs (6). The role of Tregs is of interest in the field of human disease pathogenesis, including skin cancer. Depletion of CD4+CD25+ Tregs results in enhanced immune responses against alloantigens and tumour antigens (7, 8). On the other hand, the high frequency of Tregs in patients with carcinomas reportedly contributes to lymphocyte dysfunction, leading to the suppression of anti-tumour immune responses (9). Tregs are thus inextricably connected with immune-tolerance and suppressed recognition of tumour antigens in tumour progression and recurrence. Together with Tregs, immunosuppressive macrophages, such as myeloid derived suppressor cells (MDSCs) and tumour-associated macrophages (TAMs), contribute to establishing the tumour microenvironment in skin cancer (10–12).
In parallel with immunosuppressive lymphocytes, the evaluation of effector cells and immunomodulatory cells is essential for cancer immunology. IL-27, a member of the IL-12 family, is composed of EBI3 and p35 subunits and activates both STAT1 and STAT3 through a distinct IL-27 receptor, which consists of the unique receptor subunit WSX-1 paired with the gp130 chain (13). IL-27 is produced mainly by activated antigen-presenting cells (APCs) and is involved in priming T helper 1 (Th1) cells (13–16). In addition, previous reports have also suggested that the expression of STAT1 on tumour cells is connected with tumour formation, and is frequently lost during cancer progression (17, 18).
In this report, we examined the compartmentalization of CD3+ Foxp3+ Tregs, CD163+ macrophages, and IL-27-producing cells in KA and SCC, and the expression of phosphorylated signal transducer and activator of transcription (pSTAT1) on both types of tumour cells.
MATERIAL AND METHODS
Reagents
We used the following antibodies (Abs) for immunohistochemical staining (Table SI; available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1597): mouse monoclonal Abs for human CD3 (Nichirei, Tokyo, Japan), human CD4 (Nichirei), human CD8 (Dako A/S, Glostrop, Denmark), human CD68 (Dako A/S), human CD163 (Novocastra, UK), human TIA-1 (Abcam, Tokyo, Japan), rabbit monoclonal Abs for human pSTAT1 (Cell Signaling Technology, Tokyo, Japan), rabbit polyclonal Abs for human MMP-9 (Abcam), human IL-27 (LSBio, Seattle, WA, USA) and human Foxp3 Ab (BioLegend, San Diego, CA, USA) and immunoglobulin (Ig) from an unimmunized rabbit (BioLegend). Mouse IgG1, IgG2b, and IgG2a isotype controls were obtained from R&D Systems (Minneapolis, MN, USA).
Tissue samples and immunohistochemical staining
Archival formalin-fixed paraffin-embedded skin specimens were collected from 10 patients with proliferative phase of KA and 18 patients with well- or moderately-differentiated cutaneous SCC treated in the Department of Dermatology at Tohoku University Graduate School of Medicine (Table SII; available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1597). We defined the proliferative phase of KA by typical clinical features and histological characteristics, such as symmetrical appearance with a centrally located, keratin-filled crater with overhanging epithelial lips or shoulders, and epithelial strands with ground-glass appearance penetrating the dermis from the centre of the lesions. We defined differentiation of SCC by the frequency of keratinization of tumour cells, as reported previously (1) (>75% as well-differentiated SCC, between 25% and 75% as moderately-differentiated SCC). The 10 keratoacanthoma samples and 18 SCC samples were processed for single staining of CD3, CD4, CD8, CD68, CD163, TIA-1, MMP-9, and IL-27, and developed with liquid permanent red (DAKO), 3,3’-diaminobenzidine tetrahydrochloride (Wako Pure Chemical Industries, Osaka, Japan), or double staining of Foxp3 and CD3, as described previously (19, 20). Briefly, formalin-fixed paraffin-embedded tissue samples were sectioned at 4 mm and deparaffinized. After autoclaving for antigen retrieval treatment, the sections were blocked with goat serum for 10 min, then exposed to primary antibodies at 4ºC overnight. Antibody binding was demonstrated via alkaline phosphatase-conjugated anti-rabbit Ig (Histofine SAB-AP(R) kit; Nichirei) for anti-Foxp3 Ab or immunoglobulin from an unimmunized rabbit, and via peroxidase-conjugated anti-mouse Ig (Histofine SAB-PO(M) kits; Nichirei) for the anti-CD3 or their isotype controls. Anti-Foxp3 Ab was developed with liquid permanent red.
Assessment of immunohistochemical staining
Staining of infiltrated lymphocytes was examined in more than 5 random, representative fields from each section. The number of immunoreactive cells was counted using an ocular grid of 1 cm2 at a magnification of × 400. Treg cell fractions were defined as the ratio (%) of Foxp3+ cells among CD3+ cell populations. Data are expressed as the mean ± standard deviation for Treg fractions in each skin disorder.
Statistical analysis
For a single comparison of 2 groups, Student’s t-test was used. The level of significance was set at p = 0.05.
RESULTS
CD4, CD8, TIA-1 and CD68 in peritumoural areas
First, to compare the profiles of tumour-infiltrating lymphocytes between KA and SCC, we employed immunohistochemical staining for CD3, CD4 (Fig. S1 A, B; available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1597), CD8 (Fig. S1 C, D), TIA-1 (Fig S1 E, F) and CD68 (Fig. S1 G, H). There was no significant difference in the number of these effector cells in the peritumoural areas of KA and SCC.
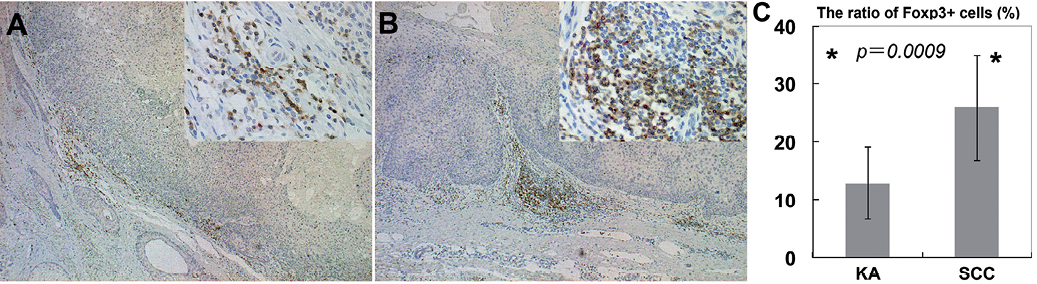
Fig. 1. Foxp3+ T cells in keratoacanthoma (KA) and squamous cell carcinoma (SCC): percentages of Foxp3+ cells among CD3+ cells in lesional skin of KA and SCC. Paraffin-embedded tissue samples from patients with KA and SCC were deparaffinized and stained using a combination of anti-Foxp3 Ab and anti-CD3 Ab (Original magnification: A, B: main ×50; right upper × 400). Sections were developed with liquid permanent red for Foxp3 (red) and with 3,3’-diaminobenzidine tetrahydrochloride for CD3 (brown). The Treg fraction among CD3+ cells was defined as the ratio (%) of CD3+Foxp3+ cells to the total number of CD3+ cells in each field, respectively. Data are expressed as mean ± standard deviation of Treg fractions in each skin disorder. *p < 0.05. (KA: n = 10, SCC: n = 18).
Foxp3+ T cells among CD3+ populations
Next, to examine the immunosuppressive cells in KA and SCC, we evaluated the percentages of Foxp3+ cells among CD3+ cell populations. We performed immunohistochemical staining of Foxp3, together with CD3 in 10 samples from patients with KA and 18 samples from patients with SCC. Immunohistochemical staining showed infiltration of substantial numbers of Foxp3+ cells in the lesional skin of KA and SCC (Fig. 1A, B). The percentages of Foxp3+ cells in KA and SCC were 12.8 ± 6.2% and 25.6 ± 9.1% among CD3+ cells (Fig. 1C). As we have reported previously, almost no Tregs are present in normal skin (14). The percentages of Foxp3+ cells among the CD3+ populations were significantly lower in KA than in SCC (p = 0.0009).
CD163+ macrophages and MMP-9+ cells
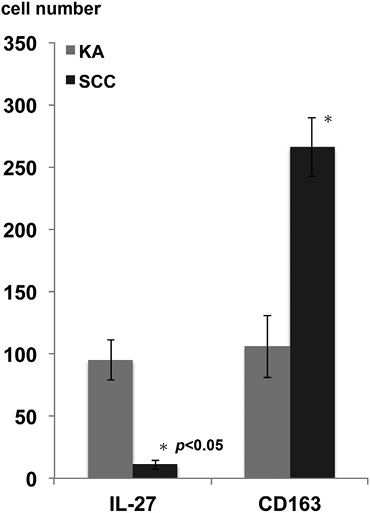
To further investigate the profiles of immunosuppressive cells around the tumours in KA and SCC, we performed immunohistochemical staining of CD163 (Fig. 2A, B) and MMP-9 (Fig. 2C, D). In SCC, dense CD163+ macrophages and MMP-9+ cells were detected throughout the dermis. In contrast to SCC, fewer numbers of CD163+ macrophages or MMP-9+ cells were observed around the tumour in KA. The numbers of CD163+ cells were significantly lower in KA than in SCC (SCC vs. KA; 266.7 ± 23.7 vs. 105.7 ± 25) (p < 0.05) (see Fig. 3)
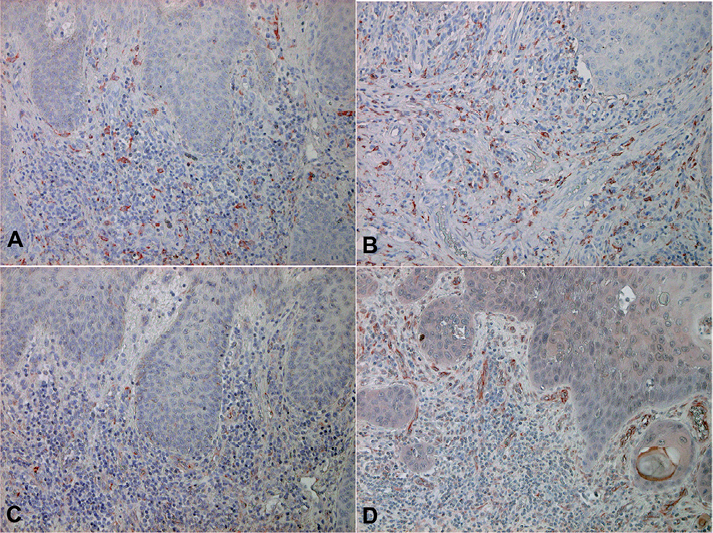
Fig. 2. Anti-CD163 and anti-MMP-9 antibody staining of keratoacanthoma (KA) and squamous cell carcinoma (SCC). Paraffin-embedded tissue samples from patients with (A, C) KA and (B, D) SCC were deparaffinized and stained with (A, B) anti-CD163 Ab or an (C, D) anti-MMP-9Ab. Sections were developed with liquid permanent red. (CD163: staining for macrophages, MMP-9 staining for macrophages and endothelial cells) (A–D: original magnification × 200) (KA: n = 10, SCC: n = 10).
.
Fig. 3. Summary of the numbers of interleukin (IL)-27-producing cells and CD163+ cells in keratoacanthoma (KA) and cutaneous squamous cell carcinoma (SCC). Five representative fields of each section were selected from both the epidermis and the dermis associated with dense dermal lymphoid infiltrate. The number of immunoreactive cells was counted using an ocular grid of 1 cm2 at a magnification of × 400. The data are expressed as the means ± standard deviation of the numbers in each area. (KA: n = 10, SCC: n = 10).
Expression of IL-27 and phosphorylated signal transducer and activator of transcription (pSTAT1)
To confirm the immunological difference between KA and SCC, we investigated further the cytokine profiles, focusing on Th1 cytokines as we previously reported (21, 22). Although we detected increased numbers of interferon (IFN)-γ producing cells in both KA and SCC (data not shown), the increased numbers of IL-27-producing cells were observed only in KA (Fig. 4A, B). Moreover, in parallel with IL-27, the expression of pSTAT1 on tumour cells was detected only in KA (Fig. 4C, D). The numbers of IL-27-producing cells were significantly higher in KA than in SCC (SCC vs. KA; 10.7 ± 3.8 vs. 94.7 ± 16) (p < 0.05) (Fig. 3).
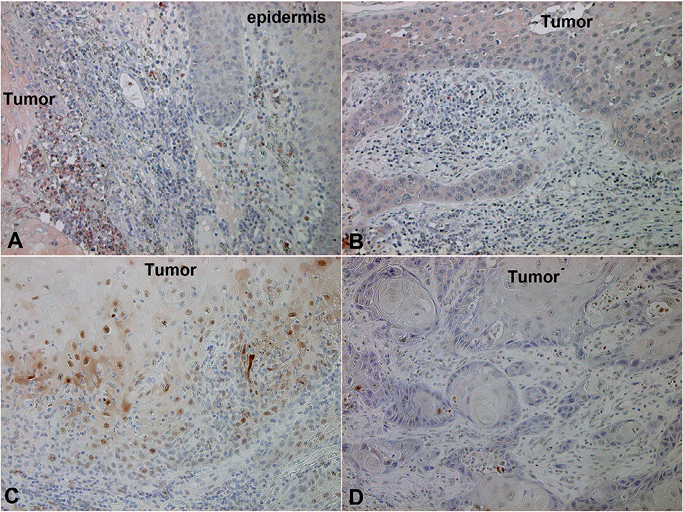
Fig. 4. Expression of IL-27 and phosphorylated signal transducer and activator of transcription (pSTAT1) on keratoacanthoma (KA) and cutaneous squamous cell carcinoma (SCC). Paraffin-embedded tissue samples from patients with (A, C) KA and (B, D) SCC were deparaffinized and stained with (A, B) anti-IL-27 Ab or (C, D) anti-pSTAT1 Ab. Sections were developed with liquid permanent red. (IL-27: staining for antigen-presenting cells, pSTAT1 staining for tumour cells) (A–D, original magnification × 200) (KA: n = 10, SCC: n = 10).
DISCUSSION
This study examined the compartmentalization of CD3+ Foxp3+ Tregs, CD163+ macrophages, and IL-27-producing cells in KA and well or moderately-differentiated SCC, and the expression of pSTAT1 on both types of tumour cells. Our results suggested the infiltration of numerous Foxp3+ Tregs around both types of tumour, but especially in SCC, and dense infiltration of CD163+ macrophages and MMP-9+ cells throughout the dermis. Interestingly, the expression of pSTAT1 was prominent in KA, and was in parallel with the increase of IL-27-producing cells.
Quantitative analysis of the expression of Foxp3+ Tregs revealed that the populations of Tregs infiltrating KA and SCC were accompanied by few or numerous CD163+ M2 macrophages and MMP-9+ cells, respectively. Together with other suppressor cells, such as myeloid-derived suppressor cells (MDSCs), tumour-associated macrophages (TAMs) and CD163+ M2 macrophages, Tregs promote an immunosuppressive environment in the tumour-bearing host (10–12, 23). In addition to directly suppressing effector T cells, Tregs also indirectly suppress effector T cells by several mechanisms through the suppression of APCs (23–25). More recently, we also demonstrated that Tregs increased the expression of B7H1 on MDSCs in a melanoma model (26). In aggregate, the increased Tregs in KA and SCC thus suggest that Tregs not only directly suppress effector T cells against tumour cells, but also affect tumour stromal cells and maintain and induce immunosuppressive cells in the tumour microenvironment.
One report has suggested that the high frequency of Tregs in patients with carcinomas contributes to lymphocyte dysfunction, leading to the suppression of anti-tumour immune responses (9), and is inextricably connected with immune-tolerance and suppressed recognition of tumour antigens in tumour progression and recurrence. Foxp3 is both necessary and sufficient for the development and function of those CD4+CD25+ Tregs (1). A recent human study suggested that the expression of Foxp3 is also induced in all CD4 T cells by antigenic stimulation (5), thus the Foxp3+ CD4 T-cell fraction includes effector cytokine-producing, non-regulatory T cells (27), and these non-regulatory Foxp3dull+ T cells are probably effector T cells (28). Indeed, in our present report, though we calculated the expression of Foxp3 only highly expressed cells, manual counting of immunoreactive cells might be limiting the data.
MMP-9 is a stromal factor that regulates the mobilization of haematopoietic stem cells from the bone marrow niche by solubilizing the membrane-bound form of c-KitL (29). Because it remodels the extracellular matrix and promotes the sprouting and growth of new blood vessels by making vascular endothelial growth factor (VEGF) available to the VEGFR-2/flk receptor on endothelial cells, MMP-9 is a linchpin in tumour progression (29). Actually, several reports revealed that the expression of MMP-9 on tumours was correlated with the progression or prognosis of several skin tumours, such as malignant melanoma, SCC, basal cell carcinoma, mycosis fungoides, extrammamary Paget’s disease and angiosarcoma (20, 29–34). In addition, other reports indicate that the expression of MMP-9 on immunosuppressive macrophages in the tumour microenvironment contributes to tumour invasion and metastasis (10, 11, 20, 34, 35). In aggregate, these reports suggest that increased numbers of MMP-9+ cells around the tumour might be connected with CD163+ M2 macrophages and contribute to the poor prognosis of tumour-bearing hosts.
In contrast to the decreased numbers of infiltrating immunosuppressive cells in KA, tumour cells highly expressed pSTAT1 and increased the numbers of IL-27-producing cells around the tumour, which suggests that the tumour microenvironment of KA is Th1-shifted. IL-27 is produced by activated APCs and induces the proliferation and expression of T-bet in naïve CD4+ T cells (28, 36). WSX-1, which was cloned as a homologue of gp130 of the IL-6 receptor, constitutes a functional, signal-transduction receptor for IL-27 with gp130. WSX-1 is highly expressed in CD4+ T cells, NK cells, NKT cells and macrophages (37). In T lymphocytes, IL-27 induces STAT1 and STAT3 activation, thus resulting in the enhancement of naïve CD4 T-cell proliferation, the promotion of early Th1 differentiation, and suppression of the differentiation of Th2 and Th17 cells (14).
In summary, the present observations contribute to understanding the role of Foxp3+ Tregs in the lesional skin of patients with KA and SCC, together with CD163+ macrophages and MMP-9+ cells (Fig. S2). Moreover, IL-27-producing cells were prominent in KA and connected to the high level of expression of pSTAT1 in KA. Since we did not directly assess the suppressive function or the cytotoxic function of these infiltrating cells in this study, and immunohistochemical analyses represent a single time-point within the life of a tumour, further analysis of the mechanisms underlying this phenomenon is needed to gain fundamental insights into the mechanisms of KA and SCC.
The authors declare no conflicts of interest.
REFERENCES
