Georgios Gaitanis and Ioannis D. Bassukas
Department of Skin and Venereal Diseases, University of Ioannina Medical School, Ioannina, Greece
Cryosurgery and topical imiquimod are established mono-therapies for superficial basal cell carcinoma (BCC) but are often insufficient for thicker BCCs. We present here a phase III, prospective, interventional, single-arm (cases only) study (trial registration: NCT01212562) to evaluate the feasibility and efficacy of cryosurgery (liquid nitrogen, open spray, 2 × 15 s; day 14) during 5 weeks’ imiquimod (“immunocryosurgery”) for primary, non-superficial BCC, ≤ 2 cm in diameter. Ninety-one consecutive patients with 134 basal cell carcinoma were evaluated. A total of 83 patients (124 tumours) started treatment, and 79 patients (119 tumours) completed at least one cycle of immunocryosurgery (feasibility: 95.2%; follow-up: 18–60 months). The efficacy after one treatment cycle was 95 ± 2% stable complete remissions (116/119 tumours cleared, 3/116 tumours relapsed: 6 treatment “failures”). Neither tumour size (p = 0.865) nor localization (p = 0.233) predicted outcome. Repeat immunocryosurgery controlled 5/6 treatment failures (overall efficacy: 99%). Lack of a conventionally treated control group is a limitation of this study. However, the results show a high therapeutic efficacy of immunocryosurgery in a large series of primary non-superficial BCC. Key words: basal cell carcinoma; cryosurgery; imiquimod; immunocryosurgery.
Accepted Jan 19, 2013; Epub ahead of print May 27, 2013
Acta Derm Venereol 2013; 93: XX–XX.
Ioannis D. Bassukas, Department of Skin and Venereal Diseases, University of Ioannina Medical School, GR-45110 Ioannina, Greece. E-mail: ibassuka@cc.uoi.gr
Basal cell carcinoma (BCC) is the most common cancer in humans, with increasing incidence worldwide, especially among Caucasians (1–3). BCC rarely metastasizes, but can cause extensive local destruction if left untreated. Consistent treatment of all diagnosed tumours is important in order to prevent development of neglected primary cases with poorer prognosis or extensive recurrences in multi-morbid elderly patients. Failure to achieve such treatment, and the difficulty of treating multiple, sometimes confluent, lesions, constitute the main therapeutic challenges (4). The current gold standard therapy for “difficult-to-treat” primary and relapsed BCC is micrographic (Mohs) surgery, which has overall 5-year recurrence rates of 0.8–1.7% in specialized centres (4). More commonly used modalities, such as (i) conventional surgery with predefined excision margins and selected pathological tissue sectioning or (ii) cryosurgery, are generally reported to be associated with higher recurrence rates (2–19%) (4, 5).
In view of the increasing incidence rates of BCC, minimally invasive and non-destructive modalities, such as local immunotherapy with interferon-α2β (6) or topical imiquimod, have been evaluated for the treatment of selected cases. To date, imiquimod has been evaluated mainly in superficial BCC (7). For nodular BCC, the highest tumour control rate (76%) with imiquimod has been reported for the most intensive application scheme tested, i.e. once daily for 12 weeks (8). Systemic therapies targeting the decisive hedgehog signalling pathway are under clinical evaluation (9, 10).
We pioneered the combination of cryosurgery with topical imiquimod application (known as “immunocryosurgery”) for the treatment of invasive BCC in an earlier pilot study (11). In addition, by modifying this method we have treated BCC cases that are difficult to treat with surgical removal (12), including periocular lesions (13), a localization where, according to some studies, Mohs surgery is associated with distinctly increased relapse rates (14). Moreover, our earlier hypothesis (15) is supported by the results of a prospective clinical comparative study, which show that tumour clearance rates of > 90% can be achieved by applying cryosurgery during, and not before, imiquimod treatment (16).
The objective of this phase III clinical trial was to evaluate the feasibility and efficacy of one cryosurgery session during ongoing daily 5-week imiquimod application, as a first-line treatment for non-superficial BCC.
PATIENTS AND METHODS
Patients
Institutional (University Hospital of Ioannina) Ethics and Clinical Trial Review Committee permission was granted according to the principles of the Declaration of Helsinki. Patient’s informed consent was given prior to therapy. During the recruitment period consecutive patients with biopsy-verified, non-superficial BCC (including nodular, micronodular, basosquamous and morphea-like BCC) were informed about immunocryosurgery as an alternative to standard surgical excision for their tumours. Inclusion criteria for the study were: age > 18 years, tumour size ≤ 2 cm (Τ1N0M0 tumours), total number of tumours n ≤ 5 at evaluation, and distance from the eyelids and mouth >1 cm. Superficial BCC, i.e. tumours presenting in biopsy multiple islands of basaloid cells attached to the under-surface of the epidermis, not extending beyond the papillary dermis (17), were excluded from this study. There were no additional exclusion criteria, with the exception of known hypersensitivity to imiquimod or cryosurgery. The intention-to-treat (ITT) population consisted of all patients who initiated treatment under the study procedure. The per-protocol population included all patients who completed treatment according to the study protocol. This phase III interventional study was registered at www.clinicaltrials.gov (NCT01212562).
Treatment protocol
Immunocryosurgery was performed as described previously (11, 16). Briefly, the patient was instructed to apply imiquimod every night on each tumour and a skin zone of approximately 0.5 cm around the macroscopic tumour margins. Two weeks after the start of treatment with daily imiquimod, a session of cryosurgery (liquid nitrogen, open spray, 2 freeze-thaw cycles, 15 s of effective freezing time each, i.e. maintenance of the state of complete freezing for 15 s after having achieved maximal freezing condition) was applied to the skin area of the tumour and a 0.5-cm rim around it. The patient was instructed to continue topical imiquimod for a further 3 weeks without interruption, resulting in a total treatment duration of 5 weeks. All “treatment failures” (for definitions see below) were scheduled to be treated with repeated 5-week courses of immunocryosurgery (up to a total of 3 courses) and, in case of further treatment failure, with surgery.
Consensus determination of the “burden of surgery”
Surgery was not a therapy option in this study; however, it is still the most widely used modality for the treatment of BCC. The expected burden of the surgery that would be required for the treatment of the tumours recruited to this study was determined in an a posteriori 2-step specialists’ consensus procedure. In the first step, based on tumour characteristics (baseline photograph, size, histology, presence of multiple synchronous tumours) and patients’ demographics and general health state, 2 specialists (a plastic surgeon and a dermatologist-surgeon) independently categorized each BCC according to the need for tumour surgery with the following procedures: (a) simple excision and primary closure, (b) small local flap, (c) major local flap, (d) partial thickness skin graft, (e) full thickness skin graft, (f) excision and healing by secondary intension. The degree of agreement of the 2 independent judgements was assessed using kappa-test statistic. Provided that the opinions of the 2 experts did not differ significantly from each other, they then jointly evaluated all BCC according to the above categories of surgical procedures.
Measures of study efficacy and statistical analyses
All outcome measures were carried out by the authors, and histology was performed when the existence of remaining or relapsed tumour was clinically evident (see below). The primary outcome of the study was evaluated for treatment effectiveness and feasibility/tolerability. Immunocryosurgery is a therapeutic procedure, which, if necessary, can be applied in repeated treatment cycles on the same tumour. Analogous to surgical therapy for BCC, (i) the first cycle of immunocryosurgery corresponds to a session of conventional surgical treatment of the tumour, and (ii) treatment with repeated cycles of immunocryosurgery in the case of partial tumour responses corresponds conceptually to the Mohs surgery procedure. Accordingly, hypotheses related to treatment effectiveness were evaluated separately with respect to outcome: (i) after a single immunocryosurgery cycle; and (ii) after completion of treatment according to protocol, i.e. with a repeated 5-week immunocryosurgery cycle in order to induce tumour clearance.
The effectiveness of treatment was determined on the basis of 2 categories of “treatment failures”: (i) failure to achieve tumour clearance, and (ii) tumour relapse. (i) Tumour clearance was determined 1 month after the end of imiquimod application according to the following 3 levels: (a) complete response (CR) when, clinically, no remaining tumour nests are suspected and complete restitution (re-epithelization) of the treated skin area is observed (with the exception of treatment-associated sequelae on local skin texture; see Results below); (b) partial response (PR) when, on the evaluation day, < 25% of the original tumour were still present; (c) no response (NR) otherwise. Tumour remnants were noted as papule/s that were not obscured by persisting erythema at the time of follow-up. Outcomes other than (a) were jointly designated as “failure to achieve tumour clearance” (i.e. no tumour clearance). (ii) “Tumour relapse” was defined as biopsy-confirmed BCC recurrence contiguous with or within 1 cm of the treatment scar of a previously completely responded tumour.
The “feasibility/tolerability” of the treatment was determined according to patient’s adherence to the procedure. “Non-feasible” was a treatment that was interrupted prematurely, irrespective of the underlying reasons.
Tumour condition at the last follow-up appointment through July 2012 was considered in treatment response analysis. One primary and 3 secondary end-points were evaluated. The primary end-point, the “efficacy of the treatment”, was defined as the rate of disease-free tumour sites (complete responders without tumour relapse) at the scheduled follow-up appointments. The secondary end-points included: (i) feasibility of the treatment, defined as the proportion of patients that terminated the study schedule; (ii) efficacy of the treatment to induce clinical tumour clearance; (iii) impact of potential predictor factors (tumour size and anatomical localization) on treatment outcome.
A dichotomized primary outcome variable was applied (treatment failure or not) and time-to-treatment failure (TTF, in months) was calculated from the end of treatment to the diagnosis of treatment failure. Similarly, durations of censored observations were defined for each patient/tumour from the end of treatment to the date of last registered follow-up appointment (or patient death). Kaplan–Meier curves were calculated to describe the distribution of TTFs, and 2-sided 95% confidence intervals were determined for the fractions of tumours in remission in each prognostic group, using the Kaplan–Meier and the life-table methods. The impact of potential prognostic factors (tumour size and anatomical localization) on the distribution of TTFs were calculated using log-rank (Mantel–Cox) analysis.
Continuous data were summarized using descriptive statistics, and categorical data were summarized using frequency counts and percentages. Fisher’s exact test or χ2 statistic was used for the comparison of measured and expected frequency distributions of categorical data. The degree of inter-observational agreement was calculated using the kappa-test. Two-sided p-values ≤ 0.05 were considered to indicate statistical significance. SPSS 19.0 edition (Chicago, IL, USA) was used for statistical calculations.
RESULTS
Fig. S1 (available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1609) shows the study population and the core study outcomes. Between 1 April 2007 and 31 March 2010 a total of 91 patients with 134 biopsy-verified BCC ≤ 2 cm in maximal clinical diameter were evaluated for treatment with immunocryosurgery. Six patients did not fulfil the recruitment criteria and were initially evaluated in error, and 2 withdrew their consent to participate. Four of the remaining 83 patients (4.8%) who initiated treatment (ITT population) did not comply with major protocol prerequisites (e.g. they skipped imiquimod applications, did not attend the scheduled cryosurgery appointment, or interrupted imiquimod application after cryosurgery) and were excluded from the study. A total of 79 of the recruited patients (95.2% of the ITT population) completed at least one cycle of immunocryosurgery according to the study protocol (per-protocol population: 40 men, 39 women; mean age: 72.4 years) in a total of 119 tumours (1–4 tumours per patient; Table I).
Table I. Demographics and baseline characteristics of the 79 patients who completed one cycle of immunocryosurgery. Note: no patients with 5 simultaneous tumours were recruited
|
Attribute |
Patients, n (%) |
|
Gender |
|
|
Female |
39 (49.3) |
|
Male |
40 (50.7) |
|
Age, years |
|
|
Range (mean ± SD) [median] |
47–92 (73.8 ± 8.9) [74.5] |
|
≤ 70 years |
31 (39.2) |
|
> 70 years |
48 (60.8) |
|
Treated tumours/patient |
|
|
1 |
54 (68.4) |
|
> 1 |
25 (31.6) |
|
2 |
15 (19.0) |
|
3 |
5 (6.3) |
|
4 |
5 (6.3) |
|
5 |
0 (0.0) |
SD: standard deviation.
Baseline characteristics for the tumours are shown in Table II. Most tumours (99/119) were located on the face; half of the extrafacial ones (10/20) in the scalp and neck regions. Moreover, the facial tumours were not randomly distributed across the surface of the face: There is a statistically significant deviation (p < 0.0001; χ2 test) in the measured spatial distribution of the facial tumours recruited in this study (Table III) from that expected from a random distribution according to the relative surface contribution of the different anatomical subregions of the face (calculated after Yoon et al. (18), with corrections for the exclusion of the periocular region and for gender composition). Approximately 4/5 of the included tumours (79%) were found to have nodular histology on pretreatment biopsy; of the remainder, 9%, 6% and 6% were of morpheic, micronodular and basosquamous histology, respectively. The follow-up period for the treated tumours ranged from 18 to 60 months (mean ± standard error of the mean (SEM): 28.3 ± 1.3 months). Most of the tumours (69/119: 58%) have been followed up for ≥ 2 years.
Table III. Anatomical tumour localization of the 119 tumours with at least one completed cycle of immunocryosurgery treatment. There was no statistically significant difference in treatment outcomes for tumours of facial vs. extrafacial localization (p = 0.5878; Fisher’s exact test)
|
Skin region |
Tumoursn (%) |
Treatment failuresn |
No tumour clearancen |
Relapsen |
Recruited/expected tumours relative to % skin surfacea |
|
Facial |
99 (83.2) |
6 |
3 |
3 |
|
|
Forehead |
10 |
1 |
0 |
1 |
0.58 |
|
Temples |
13 |
1 |
0 |
1 |
1.51 |
|
Nose |
27 |
1 |
1 |
0 |
6.28 |
|
Cheek |
39 |
2 |
1 |
1 |
0.91 |
|
Perioral/nasolabial |
5 |
1 |
1 |
0 |
0.58 |
|
Ear |
5 |
0 |
0 |
0 |
0.29 |
|
Extrafacialb |
20 (16.8) |
0 |
0 |
0 |
|
aCalculated according to (18) (see text). b Scalp/neck (n = 10); Truncal (n = 8); Acral (n = 2)
Table II. Baseline tumour characteristics and treatment outcomes after one cycle of immunocryosurgery for the 119 tumours completing at least one treatment cycle
|
Characteristic |
Tumours treated n (%) |
Treatment failuresa (n) |
Partial responses (n) |
Relapses (n) |
p-valueb |
|
Patient’s gender |
|
|
|
p = 0.4314 |
|
|
Female |
58 (48.7) |
4 |
1 |
3 |
|
|
Male |
61 (51.3) |
2 |
2 |
0 |
|
|
Patient’s age at diagnosis |
|
|
p = 1.000 |
||
|
≤ 70 years |
48 (40.3) |
2 |
1 |
1 |
|
|
> 70 years |
71 (59.7) |
4 |
2 |
2 |
|
|
Follow-up (months) |
|
|
|
|
|
|
Range (mean ± SEM) |
18–60 (28.3 ± 1.3) |
||||
|
< 24, n (%) |
69 (58.0) |
2 |
2 |
0 |
p = 0.2370c |
|
≥ 24, n (%) |
50 (42.0) |
4 |
1 |
3 |
|
|
Treated tumours/patient |
|
|
|
||
|
1 |
54 (45.4) |
3 |
2 |
1 |
p = 1.000c |
|
> 1 |
65 (54.6) |
3 |
1 |
2 |
|
|
2 |
15 (25.2) |
||||
|
3 |
5 (12.6) |
||||
|
4 |
5 (16.8) |
||||
|
5 |
0 (0.0) |
||||
|
Tumour size (maximal tumour diameter) |
|
|
|||
|
Range (mean ± SD) [median] |
4–20 (10.63 ± 4.59) [10] |
||||
|
≤ 5 mm |
20 (16.8) |
0 |
0 |
0 |
|
|
> 5–10 mm |
43 (36.2) |
3 |
2 |
1 |
|
|
> 10–15 mm |
44 (37.0) |
2 |
0 |
2 |
|
|
> 15–20 mm |
12 (10.0) |
1 |
1 |
0 |
|
|
≤ 10 mm |
64 (53.8) |
3 |
2 |
1 |
p = 1.000c |
|
> 10–20 mm |
55 (46.2) |
3 |
1 |
2 |
|
|
Expected risk for relapse after surgery relative to tumour localization |
p = 1.0000 |
||||
|
Low |
51 (42.9) |
3 |
1 |
2 |
|
|
High |
68 (57.1) |
3 |
2 |
1 |
|
aTreatment failure: either no complete response or relapse. bImpact of corresponding factor on treatment outcome by Fisher’s exact test. cImpact on outcome of follow-up period < 24 vs. ≥ 24 months, single vs. multiple simultaneous tumour treatments/patient and maximal tumour diameter ≤ 10 vs. > 10 mm respectively.
SEM: standard error of the mean; SD: standard deviation.
Burden of disease
There was a high level of agreement in the categories of surgical approaches independently proposed by the 2 experts for the treatment of the present series of tumours (kappa = 0.741; p < 0.001). Fig. S2 (available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1609) presents the consensus distribution of the surgical procedures proposed a posteriori by the 2 evaluators. For 47% of the tumours, simpler surgical measures, such as “surgical excision and primary closure” or “surgical excision and healing by secondary intention” were anticipated as inadequate to treat the tumours presently recruited for immunocryosurgery.
Outcome after a single immunocryosurgery treatment cycle
Patients’ recruitment history and core study outcome data are summarized in Fig. S1 and Table II. A total of 79 patients, 119 tumours, completed one cycle of immunocryosurgery. Typical treatment examples are shown in Figs 1 and S3 (available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1609). “Tumour clearance” was induced in 116/119 tumours (97.5%) after one cycle of immunocryosurgery; in 3 cases only partial response was achieved. Of these, 3/116 completely cleared tumours (2.5%) relapsed during follow-up, all within the first year after treatment (at 3, 6 and 12 months, respectively). Thus, the overall effectiveness of one cycle of immunocryosurgery in tumours with diameter ≤ 2 cm is 113/119 (95.0 ± 2.0%; Fig. 2A). BCC size did not affect the treatment outcome, as no difference was observed in the effectiveness of immunocryosurgery for tumours ≤ 10 mm diameter (95.3% in remission) compared with those 10–20 mm diameter (94.5%; p = 0.865, log-rank Mantel–Cox test; Fig. 2B). In addition, tumour localization in skin areas known to be associated with increased risk for relapse after surgical treatment did not affect the outcome after one cycle of immunocryosurgery (Fig. 2C). Notably, immunocryosurgery tended to be relatively more effective in tumours located in so-called “higher risk” areas for relapse after surgery compared with those located elsewhere (p = 0.233, log-rank Mantel–Cox test).
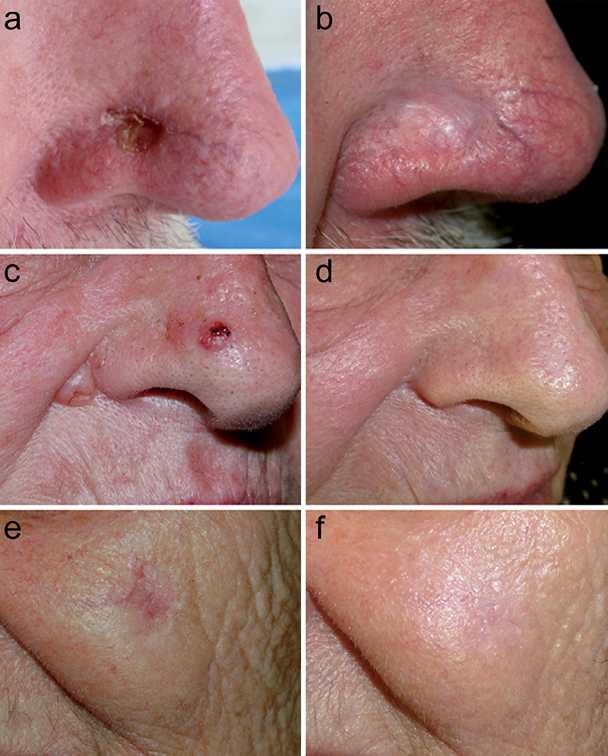
Fig. 1. Representative outcomes of the treatment of basal cell carcinoma (BCC) with immunocryosurgery. Panels A, B: 12-mm diameter tumour (65-year-old man). Panels C, D: 2 tumours, 7-mm (nose) and 10-mm (nasolabial fold) diameter (74-year-old woman). Panels E, F: 13-mm diameter tumour (82-year-old woman). Panels A, C, E: baseline; Panels B, D, F: 12-month follow-up.
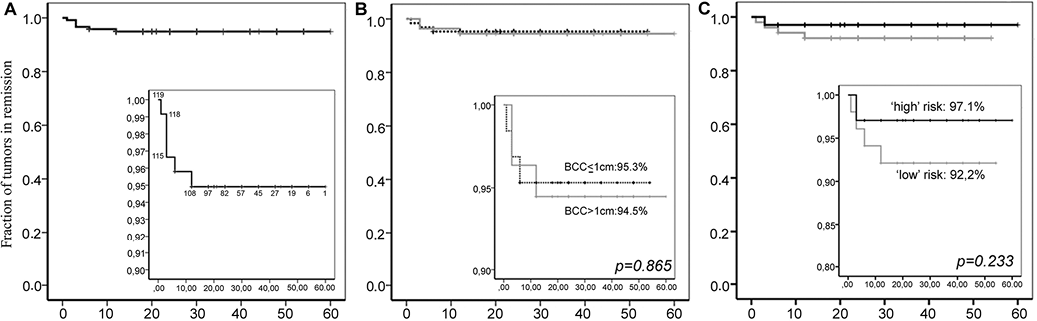
Fig. 2. Effectiveness of a single immunocryosurgery cycle. The rate of disease-free tumour sites during follow-up displayed as Kaplan–Meier curves. Panel A: overall efficacy. Panel B: comparison of the efficacy for tumours ≤ 1 cm vs. 1–2 cm in diameter. Panel C: comparison of the efficacy for tumours located in high (“H” face region) vs. low-risk regions for relapse after surgery. p-values: log-rank Mantel–Cox test results. Inserts: Detail of curves in the range of tumour remission rates 0.9–1.0 (Panels A and B) and in the range 0.8–1.0 (Panel C). Numbers in the insert in Panel A indicate the number of tumours still being followed up at the corresponding time-point.
Management of non-responders and tumour relapses
Three tumours were partial responders after the first cycle of immunocryosurgery. In all of these cases complete response was induced with additional treatment cycles (1 cycle in 2 and 2 cycles in one case). Accordingly, immunocryosurgery induced “complete response” in all 119 per-protocol treated BCC (> 99%) and the per-protocol effectiveness was 97.5% (116/119: 3 tumours relapsed).
Concerning relapsed tumours, immunocryosurgery was offered to all 3 patients with tumour recurrences during follow-up: (i) 1 tumour relapsed 3 months after treatment. Complete remission was induced with one cycle of immunocryosurgery and the tumour remained in remission after 30 months’ follow-up. (ii) The second tumour relapsed 9 months after treatment. This patient initially refused an additional cycle of immunocryosurgery and was thereafter lost to follow-up; however, the patient returned 22 months later with a relapsed tumour (diameter: 1.6 cm). At that point complete response was induced after one cycle of immunocryosurgery and the tumour remains in remission after 9 months’ follow-up. (iii) The third relapse occurred 12 months after treatment in a patient with 3 tumours all treated with immunocryosurgery. This patient refused a repeat of the immunocryosurgery cycle and has been lost to follow-up since then. Thus, taking into consideration the treatment of relapsed tumours too, the overall therapeutic effectiveness of immunocryosurgery to achieve sustained tumour control in a population of patients with BCC ≤ 2 cm maximal diameter is 99%, i.e. 118/119 of the per-protocol treated tumours.
Feasibility of immunocryosurgery and analysis of adverse events
None of the recruited patients stopped treatment due to intolerance or excessive adverse reactions. However, 4 of the initially 83 recruited patients did not adhere to the study protocol, due to poor communication or for social reasons, which corresponds to an overall feasibility in the ITT population of 95.2%.
Major complains during treatment included flu-like symptoms (anorexia, tiredness, discomfort, low-grade fever: 6 patients, 7.5%) with only 2 patients recording fever > 38°C. Symptoms associated with imiquimod treatment, such as redness, local irritation, pruritus, and oozing, peaked during the week following cryosurgery and were experienced by all patients (Fig. S3 C, H, M).
The principal adverse effects/sequels of the treatment were hypopigmentation (Fig. S3E), largely restricted to the area of the healed tumour, slight erythema that persisted for up to 3 months after treatment (Fig. S3I), and different degrees of superficial scarring that generally improved with time (compare Figs S3 D, E, I, J, N, O). A number of patients reported a peculiar, minor stinging sensation with qualities of neuropathic pain, confined to the site of the treated tumour, which lasted up to one year after treatment. No patient experienced infection at the treatment site requiring systemic antibiotic therapy.
DISCUSSION
The results of this study confirm our previous preliminary findings on the efficacy of immunocryosurgery for the treatment of non-superficial BCC (11), and highlight the usefulness of this combination procedure as an alternative treatment approach for this common skin cancer. The pathophysiological rationale of immunocryosurgery has been discussed previously (11, 15), and the significance of the timing of cryosurgery during imiquimod treatment has been shown in a recent comparative study (16). Interestingly, a recent study that evaluated the efficacy of 4-week pre-Mohs imiquimod on the burden of subsequent surgery for facial BCC points to a “periphery-to-centrum” confinement (“shrinkage”) of the tumour under this topical treatment (19). Although the mechanism underlying this finding is unknown, it may contribute to the effectiveness of immunocryosurgery, and therefore further evaluation is necessary.
Compared with the widely used surgical treatment approaches, immunocryosurgery offers overall efficacy at least comparable to that of surgery for primary tumours (97.5% vs. 97%) (5). Immunocryosurgery does not seem to be restricted by factors such as the anatomical location of BCC or the histological subtype of the tumour. Moreover, in contrast to surgery (20), wound infections are not a concern during immunocryosurgery. Finally, the burden of surgical treatment that was avoided by using immunocryosurgery in the patients in the current study is considerable: (i) 47.2% of the tumours had a maximal diameter > 10 mm; (ii) 2 experienced evaluators judged that, for 47% of the tumours, simpler surgical measures would have been insufficient for tumour treatment; (iii) more than half of the tumours (57.1%) were located in skin areas associated with increased risk for tumour relapse after surgery (Tables I and III). A study of surgery vs. immunocryosurgery for BCC is therefore required, in order to compare not only their efficacy but also their burden.
Compared with monomodal topical imiquimod, the therapeutic effectiveness of immunocryosurgery is clearly superior (discussed in (16)). For example, Butler et al. (21) employing Mohs surgery one month after completing a 6 week nightly imiquimod scheme to treat nasal BCC reported 5/12 (42%) clearance rate. In contrast, in the present study the effectiveness of immunocryosurgery to induce tumour clearance 1 month after the end of treatment was higher: 26/27( 96%) of the nasal skin tumours remained relapse-free after one treatment cycle (Table III).
The present results are in accordance with ongoing efforts to develop non-surgical approaches for the treatment of BCC (10, 22). This is important in view of the rapidly rising incidence rates of BCC and the increasing burden of surgical BCC treatment on healthcare resources (23). Currently, in addition to research into the development of new treatment modalities (10), combination strategies of established procedures are an active research field in order to improve the effectiveness of the available treatment options for BCC. The combination of 2 cycles of photodynamic therapy (PDT) with pre-treatment curettage has shown improved efficacy for < 2 cm diameter BCC (24). Furthermore, the use of imiquimod following treatment of BCC with PDT has significantly increased the efficacy of the treatment, to 75% at 10 months follow-up (25). Imiquimod after cryotherapy (cryoimmunotherapy), although therapeutically inferior to current “immunocryosurgery” for non-superficial BCC (16) has proved effective for the treatment of superficial BCC and Bowen’s disease (26), as well as for BCC refractory to prior monomodal topical imiquimod (27). Thus, immunocryosurgery, a procedure that can be offered by office-based dermatologists without access to surgery facilities, is an effective approach for the treatment of BCC, as it offers high efficacy without the need for particularly specialized training or mobilization of extensive healthcare resources.
Hypersensitivity to imiquimod or cryosurgery are the only important contra-indications in the application of immunocryosurgery to treat BCC, yet both are quite infrequent, and thus would not greatly limit the applicability of this modality in everyday practice. Due to inadequate data concerning the safety of immunocryosurgery in the immediate proximity of the eyes and lips at the time of initiation of this trial, tumours located within 1 cm of these structures were not recruited for this study. However, this is no longer an important limitation, since there is increasing data to support the safety and efficacy of immunocryosurgery for the treatment of BCC in the above localizations, although with individualized treatment schemes (13).
The most significant side-effect of this treatment was local irritation, which presented with considerable inter-individual variability that could not be predicted prior to treatment. Ongoing research in our department is evaluating the quality of life in patients before, during and after immunocryosurgery. The greatest impact on quality of life is evident during the week after cryosurgery (unpublished data). However, one month after the end of treatment, following the reduction in local irritation, successful treatment of BCC, and the generally excellent cosmetic outcome, the patients’ concerns were all resolved.
In conclusion, immunocryosurgery appears to be a feasible and highly effective treatment modality for non-superficial, primary BCC with a maximal diameter of ≤ 2 cm (T1N0M0 tumours) in low-risk areas. With the increasing prevalence of BCC it could constitute a robust, office-based alternative to standard surgical excision for these tumours irrespective of their histological type, anatomical location or patient’s general state of health. Comparative studies are now needed in order to assess the potential advantages of the present minimally invasive, non-surgical combination therapy over other, more conventional, treatment modalities.
ACKNOWLEDGEMENTS
The authors would like to thank Apostolos Gaitanis, MD, for expert advice in evaluating the burden of surgery and Areti Ganiatsa, MD, for assisting in the assessment of patients’ records.
Conflicts of interest: Both authors have received travel grants from MEDA, Hellas to present relevant data at international and local conferences.
REFERENCES
