Sven R. Quist1, Ingrid Wiswedel2, Ines Doering2, Jennifer Quist1 and Harald P. Gollnick1
1Clinic of Dermatology and Venereology, and 2Department of Pathological Biochemistry, Otto-von-Guericke University, Magdeburg, Germany
Atopic dermatitis (AD) is a multifactorial inflammatory skin disease with release of distinct inflammatory signals. This study investigated the presence of eicosanoids in AD skin and the effect of topical agents with potential to suppress inflammation. Twelve patients with moderate AD received topical treatment on either arm with tacrolimus 0.1% ointment or a lotion containing 12% ω-6 fatty acids (polyunsaturated fatty acids; PUFA) twice daily for 5 consecutive days. Interstitial fluid was collected in vivo via dermal microdialysis from 4 defined skin areas: lesional, non-lesional and topically treated skin (tacrolimus or PUFA). Markers of oxidative stress (F2-isoprostanes; 5- and 8-prostaglandin F2α) and inflammation (9α,11α-prostaglandin F2α; and prostaglandin E2) were determined by gas chromatography-mass spectrometry. All eicosanoid levels were reduced in non-lesional and tacrolimus-treated skin. A significant reduction was observed in total F2-isoprostanes; 9α,11α-prostaglandin F2α; and prostaglandin E2 in non-lesional skin and in 9α,11α-prostaglandin F2α in tacrolimus-treated compared with untreated AD skin. In conclusion, treatment with tacrolimus compared with PUFA appears to suppress eicosanoids more efficiently in AD skin. Key words: atopic dermatitis; tacrolimus; omega fatty acids; dermal microdialysis.
Accepted Feb 24, 2016; Epub ahead of print Feb 26, 2016.
Acta Derm Venereol 2016; 96: XX–XX.
Sven R. Quist, Clinic of Dermatology and Venereology, Otto-von-Guericke University Magdeburg, Leipziger Str. 44, DE-39120 Magdeburg, Germany. E-mail: squist@gmx.de
Atopic dermatitis (AD) is a chronic inflammatory, predisposed, multifactorial pruritic skin disease. The prevalence of AD has almost tripled in industrial countries during the last decade. It is associated with a disturbance of the epidermal barrier function and elevated immunoglobulin E (IgE) levels caused by sensitization to environmental allergens, and is often associated with other atopic disorders, such as asthma and allergic rhinitis (1, 2).
On the basis of a deficient cornified envelope combined with skin inflammation in patients with AD, local oxidative stress and specific immune responses to exposure to environmental allergens are induced (3, 4). An impaired skin barrier function exposed to environmental allergens, an altered immune response and release of cytokines is associated with the production of prostaglandins, such as prostaglandin E2 (PGE2) from keratinocytes, monocytes or endothelial cells in AD (5). AD pathophysiology involves increased levels of oxidative stress, mediated by the release of free radicals. In addition, 8-isoprostanes are enhanced in AD and are primarily detected when exhaled (3, 6–9).
Tacrolimus, a topical immune modulator and calcineurin inhibitor, is usually applied twice daily at a concentration of 0.1% in adults and has been used as an effective topical treatment for moderate to severe AD with proven efficacy in various clinical trials (10). However, treatment can be associated with undesired side-effects: skin burning, which occurs mainly at the beginning of treatment and pruritus, folliculitis (11, 12) as well as local immunosuppression (resulting in cutaneous bacterial, viral or fungal infections or the risk of developing cutaneous malignancies, although the last factor has not been clearly demonstrated so far), may occur (13, 14).
Although omega (ω)-6-fatty acid supplementation, mainly evening primrose or borage oil, has been used as an alternative treatment option (15–17), there is currently insufficient evidence to show beneficial effects of oral supplementation on disease severity in patients with AD. Furthermore, the beneficial effects of topical ω-6 fatty acids have not been evaluated in larger clinical trials. However, reports suggest that they may contribute to restoration of the skin barrier (18).
There are no comparative published studies on topical treatment with tacrolimus and supplementation of ω-6-fatty acids in AD and there are no reports on whether tacrolimus treatment affects eicosanoid release in AD.
Polyunsaturated fatty acids (PUFA), including ω-6 PUFA gamma linolenic acid (GLA) and linoleic acid (LA), have anti-oxidative, anti-proliferative and antibacterial properties and maintain skin barrier integrity (19, 20). PUFA shift the metabolism towards the production of more anti-inflammatory series 1 eicosanoids, such as prostaglandin E1. Therefore, PUFA may prevent the generation of pro-inflammatory series 2 eicosanoids (19).
In this study, we first tested the effect of tacrolimus and ω-fatty acids on the release of eicosanoids in vitro as a proof of concept. For this, we used irradiated HaCaT keratinocytes as a previously well-established model to study release of eicosanoids and potential inhibitors (6, 21, 22). We then used dermal microdialysis, a method whereby a semi-permeable membrane is implanted into the skin dermis and flushed with a pump to quantify the recovery of eicosanoids in vivo using an animal model and to detect the levels of mediators within the interstitial skin in vivo in patients with AD. The aim was to determine whether there is a difference in the levels of eicosanoids in the skin (clinically lesional and non-lesional skin in vivo) of AD. Finally, we determined whether topical treatment with either standard tacrolimus 0.1% or ω-6 fatty acids affected eicosanoid release in lesional skin.
MATERIALS AND METHODS (for complete details see Appendix S11)

1http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-2383
For in vitro experiments, 2×107 HaCaT keratinocytes were seeded on 25-cm Petri dishes overnight and irradiated with 100 mJ/cm2 and then exposed to 1, 10, 100 µM of either tacrolimus, ω-3 fatty acids or ω-6 fatty acids in Dulbecco‘s Modified Eagle Medium (DMEM) 10% foetal calf serum (FCS) for 24 h. Levels of 5-iso and 8-iso-PGF2α and 9α,11α-PGF2α and PGE2 in cell culture supernatants and HaCaT keratinocytes stored intracellularly were determined by gas chromatography–mass spectrometry negative-ion chemical ionization (GC/MS) (21, 24). Recovery of eicosanoids was determined by retrodialysis in vivo in anaesthetized domestic pigs (Fig. S1A–B1). Four microdialysis catheters (20 kDa cut-off membranes CMA70 60/20) were placed within the dermal porcine skin (25). Microdialysis catheters were perfused at 0.5 and 1 µl/min with 100 ng/ml of 5-iso-PGF2α, 8-iso-PGF2α, 9α,11α-PGF2α and PGE2; in NaCl 0.9% and 5% ethanol. Perfusates were replaced at 20-min intervals and the dialysates were pooled and analysed with GC/MS. For clinical microdialysis, 12 18–39-year-old patients with moderate clinically affected AD skin (minimum 10×10 cm2; SCORing Atopic Dermatitis (SCORAD) < 50) were randomized in a mono-centre clinical trial to treatment areas of either no treatment, treatment with tacrolimus 0.1% ointment (Protopic 0.1%) or 12% ω-fatty acid lotion (Eucerin 12% Omega lotion®). The patients applied the treatment twice daily for 5 days. On day 6, dermal microdialysis was performed in untreated, treated lesional and non-lesional AD skin. After flushing the 20 kDa cut-off CMA 70/20 membranes at a rate of 5 µl/min for 1 h for equilibration, all 4 membranes were perfused at a flow rate of 0.5 µl/min with NaCl 0.9%. Microdialysate samples were collected at 30-min intervals for 8 h and analysed using GC/MS as described previously (21, 24).
RESULTS
In vitro experiments
First, we investigated whether tacrolimus or ω-3 and ω-6 fatty acids affected eicosanoid release from human HaCaT keratinocytes, which has been established as a reliable in vitro model to study generation of eicosanoids and to test potential inhibitors (6, 21, 22). For this, we used the same dose of ultraviolet B (UVB) irradiation (100 mJ/cm2) to induce eicosanoid generation and treated the HaCaT keratinocytes with either tacrolimus or ω-3 and ω-6 fatty acids. We observed that tacrolimus treatment significantly and dose-dependently reduced the release of PGE2 and 9α,11α-PGF2α into the surrounding medium and intracellular 9α,11α-PGF2α and isoprostanes (which are not released into the extracellular space) (Fig. 1). ω-6 (more than ω-3) fatty acids also reduced PGE2 and 9α,11α-PGF2α levels in the surrounding medium and intracellular 9α,11α-PGF2α starting with the 1 µM dose. However, the effect of ω-6 fatty acids was much lower compared with tacrolimus. ω-6 fatty acids, but not ω-3 fatty acids, reduced isoprostane levels at a dose of 100 µM.
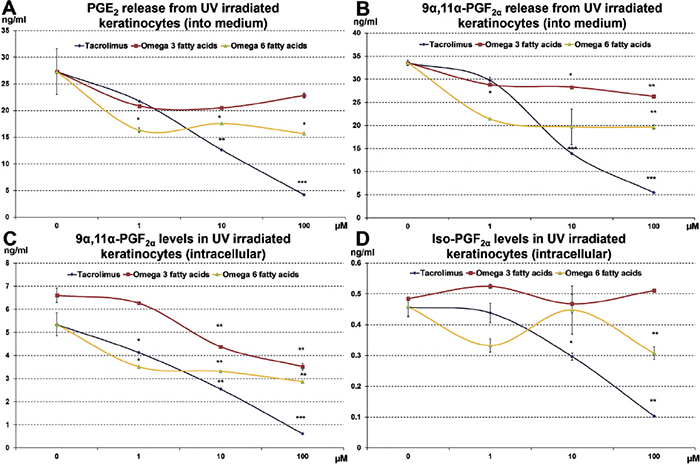
Fig. 1. Levels of prostaglandins and isoprostanes released into the supernatant (medium) or remaining intracellularly were examined in HaCaT keratinocytes following 100 mJ/cm2 ultraviolet B (UVB) irradiation with or without treatment with tacrolimus, ω-3 or ω-6 fatty acids. (*p < 0.05 indicates significance, **p < 0.01 indicates high significance, ***p < 0.001 indicates very high significance as determined by Student’s t-test).
In vivo recovery of eicosanoids in pig skin
Using microdialysis, we determined the in vivo recovery of eicosanoids in the skin, which is unknown (Fig. S11). As expected, the recovery of the small molecular weight eicosanoids through the 20 kDa microdialysis membrane was > 80% for all mediators at a flow rate of 0.5 µl/min and was consistently higher than the flow rate of 1 µl/min. Therefore, a flow rate of 0.5 µl/min was further used for clinical dermal microdialysis experiments.
Clinical microdialysis
Finally, we initiated a mono-centre clinical trial to test whether there were differences in eicosanoid release in lesional vs. non-lesional skin of AD patients and to test whether there were any inhibitory effects after treatment with topical tacrolimus 0.1% ointment or 12% ω-6 fatty acid lotion (Fig. 2). Twelve patients (mean ± SD 24.14 ± 1.89 years of age) with moderate AD (mean SCORAD 42.09 ± 1.00) (Table SI1) were enrolled in this study by simple randomization. Overall, we observed lower mean area under the curve (AUC) values for isoprostanes (5- and 8-iso-PGF2α), total isoprostanes, 9α,11α-PGF2α and PGE2 in non-lesional compared with lesional AD skin (Fig. 3). This decrease was significant for the sum of isoprostanes, 9α,11α-PGF2α and PGE2 (Table SI1) as detected by dermal microdialysis. In addition, lesional and non-lesional AD skin differed significantly for skin erythema and darkness levels. Patients with AD exhibited more skin darkness and erythema levels for lesional skin than non-lesional skin (see Fig. 3 and Table SII1).
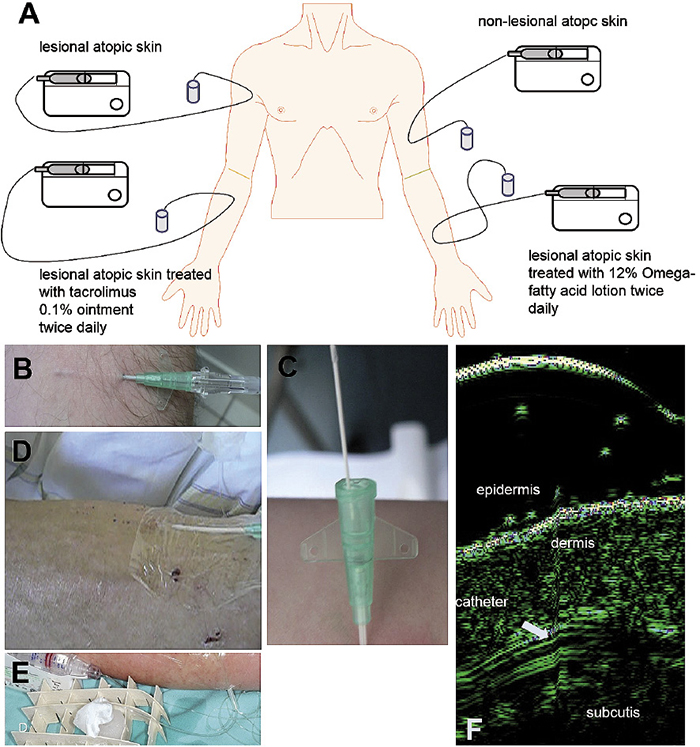
Fig. 2. Scheme of clinical microdialysis: Patients were randomized to four areas: lesional atopic skin left untreated, atopic skin treated with tacrolimus 0.1% ointment or 12% ω-fatty acid lotion for 5 days and non-lesional atopic skin (a). Introduction of CMA70 catheters via 18G i.v.-catheters (b, c) into the atopic skin were fixed with transparent self-adhesive dressings (Mepithel film©, Mölnlyke, Erkrath, Germany) (d). Microdialysate samples were kept on ice during the experiment (e). Catheter placement was guided by high resolution ultrasound (22 MHz, f).
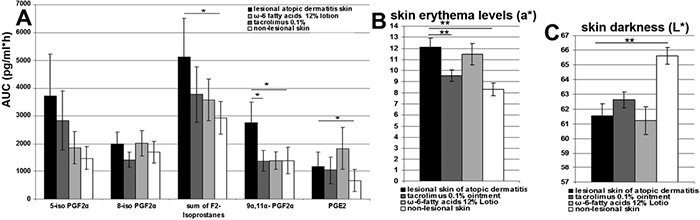
Fig. 3. (A) Mean area under the curve values (AUC) of F2-isoprostanes, 5- and 8-iso-prostaglandin F2α and prostaglandins 9α,11α-prostaglandin F2α and prostaglandin E2 (pg/ml*h) and standard deviation (SD) obtained from 12 patients with atopic dermatitis (AD). Dermal microdialysis was performed in lesional, non-lesional atopic, or atopic skin treated with 0.1% tacrolimus ointment or with 12% ω-6 fatty acid lotion (mean ± SD; *p < 0.05 indicates significance as determined by the Wilcoxon rank-sum test). Levels of erythema (B; e*>0 indicates increasing erythema) and skin darkness (C; L*, black=0, white=100) with SD from lesional, non-lesional atopic, or atopic skin treated with 0.1% tacrolimus ointment or 12% ω-6 fatty acids lotion (*p < 0.05 indicates significance, **p < 0.01 indicates high significance according to the Wilcoxon rank-sum test, comparison with untreated lesional skin).
Treatment with tacrolimus and ω-6 fatty acids reduced the mean AUC values for 5- and 8-iso-PGF2α, all isoprostanes and 9α,11α-PGF2α compared with lesional AD skin. This reduction was significant for 9α,11α-PGF2α when treated with tacrolimus 0.1% ointment. For 8-iso-PGF2α, the mean AUC value was even lower than in non-lesional skin. Tacrolimus treatment, in contrast to skin treated with ω-6 fatty acids, reduced the mean AUC value of PGE2. These results were supported by the findings of skin chromatometry. We found that tacrolimus significantly reduced skin erythema. In contrast, skin erythema reductions with ω-fatty acid treatments were much lower and skin darkness increased (Table SII1).
However, there were inter-individual differences. In 2 patients (numbers 5 and 10), treatment with ω-6 fatty acids increased the inflammatory eicosanoid release and skin erythema. These patients reported treatment failure and a burning sensation in the related treatment areas. In 2 patients (numbers 7 and 8, Table SI1) treated with tacrolimus 0.1% ointment, the levels of all eicosanoids increased, but erythema levels did not increase (Table SII1). These patients reported that the treatment failed to improve their symptoms, but that the treatment did not worsen the treated lesional skin areas.
In general, tacrolimus treatment was more effective than ω-6 fatty acid treatment regarding the decrease in eicosanoid release and skin erythema (Tables SI and SII1).
DISCUSSION
Dermal microdialysis has been used in patients with AD to study the penetration of topical drugs (31) and the effects of PGE2 on skin vasodilatation (32). Dermal microdialysis has also been used to study the effect of cyclosporine on histamine and PGD2 release in the skin of dogs (33). PGE2 and leukotriene B4 are present in biologically active and detectable concentrations in the lesional skin of patients with AD (34, 35). However, most of this research was performed decades ago, and previous experiments using the method of suction blisters to collect interstitial fluid failed to show any differences in the levels of PGE2 between lesional and non-lesional skin of patients with AD and psoriasis, but did show differences in leukotriene B4. Increased levels of 9α,11α-PGF2α have been detected in the urine of children with AD.
We used dermal microdialysis for the first time to study skin inflammation and eicosanoids in patients with AD and the possible suppressive effects of topical tacrolimus and ω-6 fatty acid treatment. We detected significant differences in the mediators of inflammation (prostaglandins) and oxidative stress (isoprostanes) between lesional and non-lesional skin and after treatment with tacrolimus 0.1% ointment, which is one of the standard treatments for patients with AD.
A limitation of this study includes the lack of a base ointment or cream of topical tacrolimus and PUFA as a control treatment. Since both galenic bases are very different, and due to limited treatment areas, they were not included.
There are 3 possible reported mechanisms through which tacrolimus could suppress the generation of prostaglandins PGE2 and 9α,11α-PGF2α. First, tacrolimus could indirectly inhibit nuclear factor of activated T cells (NFAT)-signalling through binding to immunophilins (FK-binding protein). This regulates cyclooxygenase-2 (36, 37). Secondly, tacrolimus could directly inhibit cyclooxygenase-1 (38). Lastly, tacrolimus could inhibit the pro-inflammatory cytokines interleukin (IL)-1β (39) and tumour necrosis factor alpha (TNF-α) (40), which would otherwise stimulate the release of prostaglandins, through DNA-binding to modify their transcription.
The effects of tacrolimus on oxidative stress have been conflicting (41, 42). Topical tacrolimus can re-pigment the skin in patients with vitiligo, which uses its anti-oxidative properties (43), and similar effects have been reported for the reduction in inflammation and oxidative stress in a myocardial infarction model in mini-pigs (42). Even though the exact mechanisms of how tacrolimus reduces oxidative stress are unknown, it might be reached by decreasing the amount of pro-inflammatory T cells.
PUFAs might be beneficial for patients with AD. In a diet-fed, AD, hairless mouse model, only supplementation with ω-6, but not ω-3, fatty acid reduced AD symptoms, such as itch-related scratching and skin inflammation. However, there is currently insufficient evidence of beneficial effects from clinical trials with evening primrose or borage oil (15–17, 44).
In patients with AD, the most important effects of ω-6 fatty acids might be the restoration of a proper skin barrier (18); the metabolic shift towards the production of anti-inflammatory series 1 eicosanoids; and the anti-oxidative properties of PUFAs (19). When ω-6 fatty acids were added to emollients for one week, similar effects on severity score, itch intensity, erythema and transepidermal water loss were observed than with topical 1% hydrocortisone (20). In this study, we observed a non-significant reduction in isoprostanes, but not in prostaglandin PGE2.
In conclusion, using dermal microdialysis, we demonstrated that the levels of eicosanoids are increased in clinically lesional skin compared with non-lesional AD skin and that treatment with tacrolimus appears to suppress the interstitial release of eicosanoids with inflammatory (prostaglandins) and pro-oxidative (isoprostanes) properties in vivo and in vitro. Together, these effects significantly reduced skin erythema. Topical treatment with ω-6-fatty acid also reduced the level of isoprostanes, but not prostaglandin PGE2.
ACKNOWLEDGEMENTS
This study was supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung; BMBF) (fund number 01ZZ0407 (NBL3-2)) granted to SRQ.
The authors declare no conflicts of interest.
REFERENCES
