Jacinthe J. E. Adriaansen, MD1, Marcel W. M. Post, PhD1, Sonja de Groot, PhD2,3, Floris W.A. van Asbeck, MD, PhD4, Janneke M. Stolwijk-Swüste, MD, PhD3, Marga Tepper, MD5 and Eline Lindeman, MD†, PhD1
From the 1Rudolf Magnus Institute of Neuroscience and Center of Excellence for Rehabilitation Medicine, University Medical Center Utrecht and De Hoogstraat Rehabilitation, Utrecht, 2Center for Human Movement Sciences, University of Groningen, University Medical Center Groningen, Groningen, 3Amsterdam Rehabilitation Research Center, Reade, Amsterdam, 4Spinal Cord Injury Department De Hoogstraat Rehabilitation, Utrecht and 5University of Groningen, University Medical Center Groningen, Department of Rehabilitation Medicine, Groningen, The Netherlands
OBJECTIVE: To assess the occurrence of secondary health conditions and their potential risk factors in persons with spinal cord injury from 1 to 5 years after discharge from initial inpatient rehabilitation.
DESIGN: Multicentre longitudinal study.
SUBJECTS: A total of 139 wheelchair-dependent persons with spinal cord injury.
Methods: The occurrence of secondary health conditions and their potential risk factors were assessed in a clinical interview with a rehabilitation physician at 1 and 5 years after discharge from inpatient rehabilitation and by a telephone interview 2 years after discharge. Self-report questionnaires were used for the assessment of musculoskeletal and neuropathic pain.
RESULTS: Neuropathic pain (83.7–92.1%), musculoskeletal pain (62.3–87.1%) and urinary tract infection (56.5–58.9%) were the most frequently reported secondary health conditions. The occurrence of several secondary health conditions was higher among women and individuals with a complete lesion, tetraplegia, and with a higher body mass index.
CONCLUSION: Secondary health conditions are common in the first years post-discharge following spinal cord injury, and their course seems to be relatively stable. These results emphasize the number of health issues that must be considered during post-injury care of persons with spinal cord injury living in the community, and the importance of a well- coordinated interdisciplinary approach from specialized rehabilitation centres.
Key words: spinal cord injury; secondary health conditions; risk factors; rehabilitation; longitudinal.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Marcel W. M. Post, De Hoogstraat Rehabilitation, PO Box 85238, 3508 AE Utrecht, The Netherlands. E-mail address: m.post@dehoogstraat.nl
Accepted Apr 15, 2013; Epub ahead of print Oct 3, 2013
Introduction
Persons with spinal cord injury (SCI) often face serious health problems, such as bladder and bowel disorders, pressure ulcers and neuropathic pain. These secondary health conditions (SHCs) have been defined as: physical or psychological health conditions that are influenced directly or indirectly by the presence of a disability or underlying physical impairment (1). SHCs hamper an active lifestyle and quality of life on top of the primary motor and sensory impairments due to the SCI (2). They are a frequent cause of mortality and rehospitalizations (3–6).
Few prospective studies have provided a longitudinal perspective on the occurrence of SHCs in persons with SCI (7–9). Other studies have compared the occurrence of SHCs between groups with different times since injury (TSI), but in a cross-sectional design (10–14).
One study is available in which persons with SCI participated in telephone interviews on SHCs at their first, third and fifth year post-injury (7). The most frequently reported SHCs were problematic spasticity (34%, 31% and 28%, respectively) and musculoskeletal pain (28%, 29% and 36%, respectively). However, assessment of SHCs by self-report was a limitation of this study.
Some other studies collected their data through objective assessments (8, 9, 11, 12). Two of these studies utilized the National Spinal Cord Injuries Statistical Center (NSCISC) (8, 11). The NSCISC collects data through a combination of annual medical history interviews and physical examinations. The 2 studies reported on different SHCs. In the longitudinal study (8), with a TSI of 5 years, the most frequently reported SHCs were constipation (40.0%), bowel accidents (35.3%) and upper-extremity pain (34.1%). With an occurrence of 15.2% at the first year, 17.8% at the second year, and 19.9% at the fifth year post-injury, pressure ulcers were the most frequently reported SHC in the retrospective study (11).
Risk factors for SHCs in the first years post-injury, in particular SCI-related, have been investigated by previous studies. Not surprisingly, persons with a complete lesion and tetraplegia are most at risk for many SHCs (8–13, 15, 16). Higher age (9, 11, 16), female gender (9, 12, 13), male gender (9, 11, 12), higher body mass index (BMI) (9), traumatic injury (9) and smoking (9) are other identified risk factors.
Although differing in design and used methods these studies emphasize the number of health issues that must be considered during the post-injury care.
The Dutch research programme on the restoration of mobility, “The Umbrella Project”, a prospective cohort study (17), provided the first objective information on the occurrence, course and risk factors of SHCs in the Netherlands, from the start of inpatient rehabilitation until 1 year after discharge from inpatient rehabilitation (9). During that follow-up period most participants experienced neuropathic and musculoskeletal pain, or spasticity. Increased age, higher BMI, traumatic lesion, tetraplegia and complete lesion were identified as risk factors for SHCs. Knowledge on the course of SHCs during a longer follow-up period is, however, needed to develop preventive strategies and to improve treatment programmes for SHCs.
The present study assesses the occurrence of SHCs using data from The Umbrella Project at 1, 2 and 5 years after discharge from inpatient rehabilitation. Research questions were:
• What is the occurrence of SHCs (pressure ulcers, heterotopic ossification, urinary tract infection (UTI), pulmonary infection, autonomic dysreflexia (AD), hypotension, oedema, problematic spasticity, neuropathic pain, musculoskeletal pain and cardiovascular disorders) in persons with SCI 1, 2 and 5 years after discharge from inpatient rehabilitation?
• Are demographic (age, gender), lifestyle (smoking, body mass index), or SCI-related (aetiology, level, completeness) variables risk factors for these SHCs?
Methods
Details on The Umbrella Project are provided elsewhere (17). In short: persons were recruited in all 8 rehabilitation centres with specialized SCI units in the Netherlands between August 2000 and July 2003. Inclusion criteria were: (i) recently acquired SCI; (ii) age between 18 and 65 years; (iii) grade A, B, C, or D on the American Spinal Injury Association (ASIA) Impairment Scale (AIS); (iv) sufficient understanding of the Dutch language; and (v) expected to remain wheelchair-dependent, at least for longer distances. This choice was made because The Umbrella Project was focused on restoration of wheeled mobility, and the core physical tests were to be performed in a wheelchair. Exclusion criteria were: (i) a SCI caused by a progressive disease (e.g. malignant tumour, multiple sclerosis); (ii) a progressive disease (e.g. Parkinson's disease, progressive neuromuscular disorder); and (iii) psychiatric problems.
Approval of the research protocol was obtained from the Medical Ethics Committee of the SRL/iRv Hoensbroeck and the Medical Ethics Committee of the University Medical Center Utrecht. All participants gave written informed consent prior to participation.
Procedure
Data from 3 measurement occasions were used for the present study: 1 year (T1), 2 years (T2) and 5 years (T3) after discharge from inpatient rehabilitation.
The measurements relevant for this study comprised a consultation by a SCI rehabilitation physician, including a physical examination at T1 and T3, a structured telephone interview by a trained research assistant at T2, and a self-report questionnaire for the assessment of neuropathic pain and lifestyle habits at all 3 measurement occasions. A self-report questionnaire on musculoskeletal pain was only included at T1 and T3. Assessment of the occurrence of SHCs was part of the consultation at T1 and T3 and part of the telephone interview at T2.
Secondary health conditions
The occurrence of a SHC was assessed over the last 12 months and reported as 0 = no occurrence over the last 12 months, or 1 = currently present or has occurred in the last 12 months. The SHCs assessed this way were: pressure ulcers, problematic spasticity, UTIs, pulmonary infections, heterotopic ossification, oedema, hypotension, AD, musculoskeletal and neuropathic pain. Cardiovascular disorders were grouped and included conditions such as myocardial infarction or aortic valve stenosis.
When a participant indicated that he or she had a pressure ulcer in the past 12 months, the rehabilitation physician asked further questions on the location and the severity of the ulcer according to the classification of the European Pressure Ulcer Advisory Panel (EPUAP): grade I, II, III or IV (18). All pressure ulcers, irrespective of grade, were included.
The occurrence of problematic spasticity during certain activities was assessed for: sleeping, the execution of a transfer, washing oneself and clothing, wheelchair propulsion, and other daily activities. Answers were registered as follows: 0 = no discomfort caused by spasticity, 1 = some discomfort caused by spasticity, and 2 = much discomfort caused by spasticity. Problematic spasticity was registered when a participant scored much discomfort caused by spasticity for at least one activity.
UTI was operationalized as a symptomatic UTI (e.g. fever, malaise, incontinence, increased spasms of legs, abdomen or bladder, gritty particles or mucus in the urine or cloudy urine, foul-smelling urine) which was treated with antibiotics. Pulmonary infections were also only included when they were treated with antibiotics.
Heterotopic ossification was defined as the presence of bone in soft tissue surrounding paralysed joints confirmed by radiological examination.
Oedema was scored when the participant had received treatment (e.g. compression stockings, bandages, tubigrip, diuretic medication).
The occurrence of hypotension was checked by the assessment of symptoms (e.g. light-headedness or dizziness, fainting).
AD was defined as a sudden reaction of the autonomic nervous system triggered by a stimulus below the level of the lesion (e.g. bladder distension, UTI) which caused an increase in blood pressure associated with symptoms such as: (i) below the level of the lesion: piloerection, pallor, cool extremities, profuse sweating; (ii) above the level of the lesion: severe headaches, nasal congestion, flushing of the skin and bradycardia. When the occurrence of AD was checked a description of AD with the most common associated symptoms, as described above, was given.
Standardized questions on the nature of pain were completed when the participant reported having pain. Musculoskeletal pain was defined as nociceptive pain originating from bone, joint or muscle trauma or overuse (19). Thirteen locations on the upper and lower limbs, back and neck were scored with a 5-point scale (ranging from 1: “not severe” to 5: “very severe”). A sum score was made by adding up the scores for the 13 locations. Sum scores ranged from 1 to 65. The occurrence of musculoskeletal pain was only registered at T1 and T3. Severe musculoskeletal pain was defined as having scored severe pain or very severe pain in at least one location.
Neuropathic pain was defined as at-level or below-level pain originating from syringomyelia, spinal cord ischaemia or trauma (19). The presence (yes/no) and severity of 8 neuropathic pain characteristics (other pain than musculoskeletal pain, numbness, itching, tingling, cold, warm, girdle zone pain and phantom feeling) were assessed using a 5-point scale (ranging from 1: “not severe” to 5: “very severe”). A sum score was made by adding up the scores of all 8 neuropathic pain characteristics. Sum scores ranged from 1 to 40. Severe neuropathic pain was defined as having scored at least one characteristic as severe or very severe.
Potential risk factors
We selected potential risk factors based on Haisma et al. (9): age, gender, smoking status (smoker vs non-smoker), BMI, aetiology (traumatic vs non-traumatic), level of the lesion (paraplegia vs tetraplegia) and completeness of the lesion (motor complete vs motor incomplete). Tetraplegia was defined as a lesion at or above the first thoracic segment, and paraplegia as a lesion below the first thoracic segment. A complete lesion was diagnosed in the absence of motor and sensory function in the sacral segments, i.e. ASIA Impairment Scale grade A. AIS grades B, C, and D were considered incomplete (20).The assessments of BMI, level and completeness of the lesion were all part of the physical examination performed by the rehabilitation physician at T1 and T3. Since a physical examination was not possible at T2, the data of level and completeness of the lesion were adopted from T1. At T2, height was also adopted from T1, and body weight was asked for by the research assistant to determine the person’s BMI.
Statistics
Only persons who completed at least 2 measurement occasions were included in the analyses. A non-response analysis was performed by comparing data at the start of active rehabilitation between persons who completed the measurement 5 years after discharge with persons who did not, using χ2 and t-tests.
Descriptive statistics of participants demographic and SCI characteristics were calculated for each measurement. Random coefficient analyses (MlwiN version 2.02) were used to estimate the occurrence of the SHCs and the association with the potential risk factors.
Analysis of secondary health conditions. A logistic random coefficient model was made for the occurrence of each SHC. Time was included as a set of 2 dummy variables with T2 as reference. The occurrence of a SHC at T2 was estimated by the intercept: 1/{1 + exp [ – (intercept)]}. The occurrence of a SHC at the other 2 measurement occasions was estimated as: 1/{1 + exp [ – (intercept + regression coefficient)]} (21). Significance was set at a p-value less than 0.05.
The severity scores for neuropathic pain were estimated with a linear regression model. Again, time was modelled as 2 dummy variables and the score at T2 was estimated by the intercept. The sum scores at the other measurements were calculated by adding the intercept to the regression coefficient of the dummy variable.
Analysis of potential risk factors. All risk factors were simultaneously added to the previous described models. With these multivariate models, the contribution of each risk factor was corrected for. Odds ratios (ORs) for the risk factors were calculated as: OR = exp[regression coefficient]. The corresponding 95% confidence intervals were calculated as: exp[regression coefficient ± (standard error × 1.96)].
Results
Participants
At the start of active rehabilitation, 224 persons with SCI were included in the study. A total of 156 persons participated 1 year after discharge from the rehabilitation centre (the present study’s first time of assessment), 99 persons 2 years and 146 persons 5 years after discharge. Because 2 rehabilitation centres did not participate 2 years after discharge, a lower number of participants appeared in this measurement. A total of 139 persons completed at least 2 of the 3 measurements and were included in the analyses. Participants were lost to follow-up for several reasons: 27 persons died, 18 refused to collaborate, 5 moved, 11 could not be contacted, and the rest had other reasons for dropping out of the study.
Table I gives the descriptive characteristics at the start of active rehabilitation of the participants and the non-participants in the measurement occasion 5 years after discharge. No significant differences between both groups were seen, except that the non-participants were somewhat older than the participants, and less often had a complete lesion.
|
Table I. Descriptive characteristics at the start of active rehabilitation of the participants and non-participants 5 years after discharge |
|||
|
Participants |
Non-participants |
p-value |
|
|
Participants, n |
139 |
85 |
|
|
Age, years, mean (SD) |
39 (14) |
43 (14) |
0.048 |
|
Gender, % male |
72 |
79 |
0.232 |
|
Body mass index, kg/m2, mean (SD) |
22.8 (3.9) |
22.9 (3.7) |
0.869 |
|
Smoker, % smoker |
24 |
25 |
0.793 |
|
Cause, % traumatic |
77 |
67 |
0.109 |
|
Level, % tetraplegia |
36 |
48 |
0.070 |
|
Completeness, % complete |
57 |
38 |
0.006 |
|
SD: standard deviation. |
|||
Table II gives the descriptive characteristics of the participants at all measurement occasions. Approximately one-third of the participants had a lesion at or above the first thoracic segment, and approximately half of the lesions were complete. More than three-quarters of the lesions were of traumatic origin.
|
Table II. Descriptive characteristics of the 139 participants who completed at least 2 of the 3 measurements and who were included in the analyses |
|||
|
1 year after discharge |
2 years after discharge |
5 years after discharge |
|
|
Participants, n |
139 |
98 |
121 |
|
Age, years, mean (SD) |
40 (14) |
41 (14) |
44 (13) |
|
Gender, % male |
72 |
75 |
73 |
|
Body mass index, kg/m2, mean (SD) |
24.4 (4.5) |
24.4 (4.8) |
25.6 (4.8) |
|
Smoker, % smoker |
35 |
32 |
24 |
|
Cause, % traumatic |
77 |
81 |
78 |
|
Level, % tetraplegia |
33 |
31 |
34 |
|
Completeness, % complete |
53 |
54 |
58 |
|
SD: standard deviation. |
|||
Secondary health conditions
Fig. 1 shows the estimated occurrence of a SHC at each time-point. The most frequently reported SHCs were neuropathic pain (83.7–92.1%) and UTIs (56.5–58.9%). Musculoskeletal pain was reported by 62.3% at T1 and 87.1% at T3.There was a significant decrease in the occurrence of problematic spasticity and the occurrence of neuropathic pain between 1 and 2 years after discharge.
Fig. 1. The estimated occurrence of the secondary health conditions at each time of assessment: random coefficient modelling. *Significant difference (p≤0.05) between the estimated occurrence of neuropathic pain and of problematic spasticity at 1 and 2 years after discharge from inpatient rehabilitation.
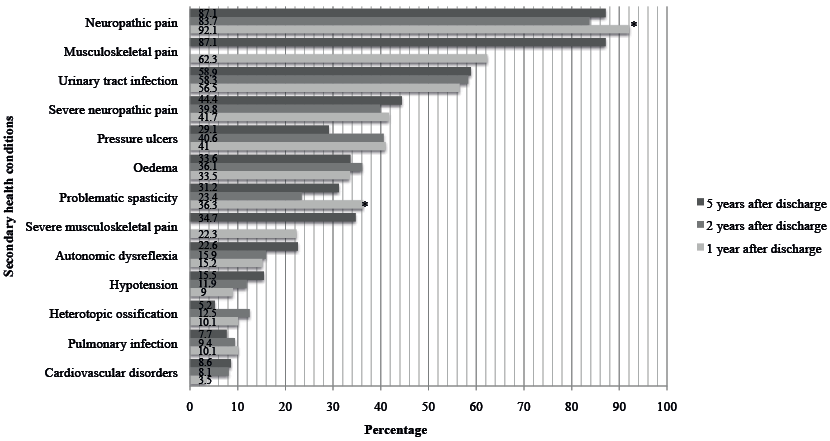
The mean severity score for neuropathic pain at T1, T2 and T3 was, respectively, 7.1, 7.6 and 9.3 points. The differences of 2.2 points between T1 and T3 and 1.7 points between T2 and T3 were significant.
Neuropathic pain was severe for 41.7% of the participants at T1, 39.8% at T2 and 44.4% at T3.
The mean severity score for musculoskeletal pain at T1 and T3 was, respectively, 7.1 and 10.1 points. This was a significant difference. Musculoskeletal pain was severe for 22.3% of the participants at T1 and 34.7% at T3.
Risk factors
Table III gives the ORs for the associations between the potential risk factors and the occurrence of a SHC. The most frequently observed significant risk factors were female gender, an increase in BMI, having a tetraplegia and having a complete lesion.
|
Table III. Odds ratios (and 95% confidence intervals (95% CI)) for the association between secondary health conditions (SHCs) and potential risk factors: multivariate logistic random coefficient modelling. Regression coefficients (and 95% CI) for the association between the severity score of neuropathic pain and potential risk factors: multivariate random coefficient modelling |
|||||||
|
Age, years OR (95% CI) |
Gendera OR (95% CI) |
Smokinga OR (95% CI) |
BMI, kg/m2 OR (95% CI) |
Causea OR (95% CI) |
Levela OR (95% CI) |
Completenessa OR (95% CI) |
|
|
Cardiovascular disorders |
1.01 (0.96–1.06) |
2.90 (0.95–8.86) |
1.66 (0.45–6.09) |
1.17 (1.04–1.32) |
0.66 (0.16–2.68) |
2.29 (0.57–9.25) |
0.70 (0.22–2.29) |
|
Pulmonary infection |
1.03 (0.99–1.07) |
2.73 (1.09–6.81) |
0.96 (0.33–2.86) |
1.10 (1.00–1.22) |
0.63 (0.18–2.22) |
0.18b (0.06–0.52) |
1.86 (0.65–5.34) |
|
Heterotopic ossification |
0.98 (0.95–1.02) |
0.30 (0.09–1.04) |
0.55 (0.21–1.42) |
0.94 (0.86–1.04) |
0.70 (0.17–2.82) |
0.87 (0.35–2.15) |
1.56 (0.62–3.88) |
|
Hypotension |
0.99 (0.95–1.03) |
2.78 (1.16–6.66) |
1.04 (0.41–2.60) |
0.99 (0.91–1.08) |
1.24 (0.34–4.54) |
0.09 (0.03–0.23) |
1.31 (0.52–3.33) |
|
Autonomic dysreflexia |
0.99 (0.96–1.01) |
0.94 (0.45–1.96) |
0.64 (0.29–1.40) |
1.09 (1.02–1.16) |
1.23 (0.47–3.19) |
0.20 (0.10–0.42) |
3.09 (1.43–6.67) |
|
Oedema |
1.03 (1.01–1.06) |
2.14 (1.19–3.85) |
0.70 (0.381–1.28) |
1.10 (1.03–1.16) |
1.35 (0.66–2.77) |
0.49 (0.27–0.90) |
3.14 (1.71–5.78) |
|
Pressure ulcers |
1.01 (0.99–1.03) |
1.48 (0.76–2.56) |
0.82 (0.47–1.43) |
1.03 (0.97–1.09) |
1.42 (0.72–2.82) |
0.70 (0.40;1.23) |
3.34b (1.92–5.83) |
|
Urinary tract infection |
1.00 (0.98–1.02) |
1.58 (0.91–2.73) |
0.83 (0.49–1.40) |
1.01 (0.96–1.07) |
1.06 (0.55–2.01) |
0.69 (0.41–1.19) |
2.84 (1.70–4.75) |
|
Problematic spasticity |
0.99 (0.97–1.02) |
1.06 (0.60–1.87) |
1.06 (0.60–1.87) |
1.00 (0.94–1.06) |
1.62 (0.82–3.22) |
0.53 (0.30–0.93) |
1.14 (0.66–1.98) |
|
Neuropathic pain |
1.04 (1.00–1.07) |
1.59 (0.60–4.20) |
0.86 (0.37–2.00) |
0.98 (0.90–1.07) |
3.60 (0.76–17.10) |
0.34 (0.13–0.89) |
1.22 (0.54–2.74) |
|
Musculoskeletal pain |
0.99 (0.97–1.02) |
1.86 (0.91–3.83) |
0.90 (0.64–1.28) |
1.11 (1.03–1.20) |
0.83 (0.37–1.88) |
0.98 (0.49–1.94) |
0.76 (0.40–1.47) |
|
Neuropathic pain severity scorec |
0.06 (0.00–0.11) |
1.65d (0.21–3.09) |
–1.28 (–2.73–0.17) |
0.10 (–0.05–0.25) |
–1.39 (–3.14–0.35) |
–0.89 (–2.31–0.53) |
–0.35 (–1.73–1.04) |
|
Musculoskeletal pain severity scorec |
–0.03 (–0.12–0.06) |
2.67 (0.33–5.01) |
–0.838 (–3.33–1.66) |
0.07 (–0.18–0.32) |
2.51 (–0.26–5.28) |
–1.12 (–3.53–1.30) |
–2.28 (–4.64–0.08) |
|
Significant associations (p ≤ 0.05) are printed in bold. aGender: men = 0, women = 1; Smoking: non-smoker = 0, smoker = 1; Cause: traumatic = 0; non-traumatic = 1; Level: tetraplegia = 0, paraplegia = 1; Completeness: incomplete = 0, complete = 1. bAs an example is given that persons with a complete lesion were 3.3 times more at risk of a pressure ulcer, than those with an incomplete lesion; persons with a paraplegia were 5.6 times less at risk of a pulmonary infection, than those with a tetraplegia. cRegression coefficients (and 95% CIs) are given. dAs an example is given that women scored their degree of neuropathic pain 1.7 points higher than men. |
|||||||
Persons with a complete lesion were at increased risk for pressure ulcers, UTIs, AD and oedema. Females had, compared with males, an increased risk for the occurrence of a pulmonary infection, hypotension and oedema. Persons with paraplegia were significantly less susceptible for pulmonary infections, AD, hypotension, oedema, problematic spasticity and neuropathic pain compared with persons with tetraplegia. A higher BMI was significantly associated with an increased risk of pulmonary infection, AD, oedema, musculoskeletal pain and cardiovascular disorders.
Table III also gives the regression coefficients for the association between potential risk factors and the severity score of neuropathic and musculoskeletal pain. An increase in age was associated with an increase in severity score of neuropathic pain (0.6 points per 10 years of age). Females scored their severity of neuropathic pain and musculoskeletal pain respectively 1.7 and 2.7 points higher compared with males.
Discussion
Secondary health conditions
Our study shows that at 1, 2 and 5 years after discharge from inpatient rehabilitation neuropathic pain and UTIs were most often reported by the participants. Musculoskeletal pain had a high prevalence at 1 and 5 years after discharge. However, when we took only severe complaints of neuropathic and musculoskeletal pain into consideration the rates were considerably lower. Furthermore, we found that the prevalence of SHCs was relatively stable in this period.
The present study was a sequel to the study by Haisma et al. (9), who researched the occurrence of the same SHCs during inpatient rehabilitation until 1 year after discharge. As in our study, neuropathic pain was the most frequently reported SHC, with a stable occurrence of 89–91% across measurement occasions. We also noted some differences in the occurrence of SHCs with regard to the results of Haisma et al. We observed a slight increase in the occurrence of AD, a decrease in the occurrence of heterotopic ossification and a slight increase in the occurrence of cardiovascular disorders.
Differences from other studies that assessed the occurrence of several SHCs among persons with SCI in the first years post-injury might be attributed to the use of various study designs and data collection methods; e.g. physical examinations (12), in-person interviews (11, 12), self-report questionnaires (10, 16) and telephone interviews (7, 11). Furthermore, none of these studies explored exactly the same SHCs as we did and there were differences in the selection procedure of the study population. We included relatively severely spinal cord injured persons, since they all had to be dependent on a wheelchair, at least for longer distances. Also, the period covered by our study (the previous 12 months) is likely to increase the prevalence of SHCs compared with studies using a shorter time-frame (e.g. at the time of medical examination, or in the previous 4 weeks).
Since neuropathic pain, musculoskeletal pain and UTIs were the most frequently reported SHCs we will discuss these 3 SHCs separately.
Neuropathic pain
In a longitudinal cohort study (22) at-level neuropathic pain and below-level neuropathic pain were present in, respectively, 41% and 34% of persons with traumatic SCI in the first 5 years post-injury. The latest review on the occurrence and chronicity of neuropathic pain reported a prevalence of 40% (23). Our percentages are much higher (84–92%), which might be explained by not making a distinction between at- or below-level neuropathic pain. We also included a wider range of characteristics in our definition of neuropathic pain. When only severe neuropathic pain is addressed, our numbers of occurrence (40–44%) are more in accordance with the literature. Furthermore, our results are consistent with the literature, in that those with neuropathic pain early following their injury are likely to continue to experience ongoing pain (22–24).
Musculoskeletal pain
In a study using a similar follow-up period (22), musculoskeletal pain was present in 40% of the participants at 6 months following SCI (compared with 62.3% at our first follow-up year) and in 59% of the participants 5 years after SCI (compared with 87.1% in our study). They also found that, at 5 years following SCI, 25% reported their musculoskeletal pain as severe. We found a slightly higher percentage of 34.7%. What corresponded was the increase in musculoskeletal pain during the 5-year follow-up period, which can be explained by the physiological age-related decline in the musculoskeletal system and the chronic overuse of the upper extremities due to their wheelchair-dependent life (25).
Urinary tract infections
In the present study we found an occurrence of UTIs at 1, 2 and 5 years following SCI of, respectively, 56.5%, 58.3% and 58.9%. These results are similar to earlier findings of Levi et al. (12) and Noreau et al. (10), who noted an occurrence that varied between 55% and 77% (TSI 0–7 years).
Course of secondary health conditions
Only the changes in reported neuropathic pain and problematic spasticity 2 years after discharge from inpatient rehabilitation compared with 1 year after discharge were found to be significant. This might be explained by the rather short post-injury period that was used for the follow-up measurements. Continuation of the follow-up is necessary to establish the long-term course of SHCs in this cohort.
Risk factors
We found that the occurrence of several SHCs was higher among women and individuals with a complete lesion and particularly those with tetraplegia and a higher BMI. For some SHCs this was not surprising, since there is a pathophysiological explanation for it.
First, AD can occur only in spinal cord injured persons with a lesion at or above T6 (26, 27). Therefore, it is rational that persons with paraplegia are less susceptible. It has also been described as being less frequent and less severe in incomplete lesions (26, 28). Secondly, persons with tetraplegia are more at risk for pulmonary complications, such as pulmonary infection, because these are associated with respiratory muscle paralysis, which causes, for example, impaired cough, difficulty mobilizing secretions and microatelectasis (29). Thirdly, it is known that the likelihood of experiencing (orthostatic) hypotension is higher amongst persons with higher spinal cord lesions. Sympathetic nervous system dysfunction below the level of injury (due to loss of supraspinal control) and the loss of reflex vasoconstriction are 2 major causes for hypotension following SCI (30, 31). The extent to which this sympathetic control is disrupted is directly related to the level of the lesion (30, 32).
The observed significant associations between female gender and pulmonary infections, hypotension, oedema and the severity of musculoskeletal and neuropathic pain are difficult to explain. As far as we know this also has not been described in previous studies. Our observed significant association between female gender and the severity of neuropathic pain is in contradiction with the literature on pain in persons with SCI. Cardenas et al. (33) for instance, reported no differences between gender and pain severity scores in persons with SCI. In addition, a retrospective study on neuropathic pain after traumatic SCI found no correlations with gender (15).
We found no significant differences between traumatic and non-traumatic SCI. This indicated that, adjusted for differences between age, gender, level and completeness of SCI, the occurrence of SHCs did not differ between persons with traumatic and persons with non-traumatic SCI.
Limitations
Our study was limited by the fact that only Dutch persons with a SCI between 18 and 65 years old who were wheelchair dependent (at least for longer distances) were included. This may influence the degree to which the results can be generalized to the whole population of persons with a SCI. Furthermore, the period of observation is too short to make any statement on the course of different SHCs in the long-term.
It should also be taken into account that, at T2, the results were based on a telephone interview by a trained research assistant. This is in contrast to the more objective manner of data collection by the consultation and physical examination by the rehabilitation physician at T1 and T3.
Unfortunately, we could not report data on co-morbid conditions or extra-spinal injuries, since these data were not systematically registered.
Finally, we did not correct for medication use in the logistic random coefficient models, since we had too much missing data on medication use at T3. This is unfortunate, since medication use could have had an effect on the reported SHCs, in particular for problematic spasticity and neuropathic pain.
Conclusion
These results emphasize the importance of a well-coordinated interdisciplinary approach during the follow-up care of persons with SCI living in the community. Since this kind of approach is feasible only at specialized rehabilitation centres, persons with SCI should be encouraged to contact these centres, instead of their general practitioner, in case of SCI-related health problems.
Follow-up care should consist of structured consultations with a rehabilitation physician at set times. In addition to offering adequate treatment in case of SHCs, follow-up care should also be aimed at, for example, an early identification of spinal cord injured persons at risk for certain SHCs and continued patient education on SHCs.
To be able to observe possible changing trajectory patterns of SHCs in persons ageing with SCI, studies such as these need to be conducted for more than 5 years. Future research should also be aimed at exploring which of these SHCs play a role in limiting participation, activity level and quality of life.
The new research project “Active LifestyLe Rehabilitation Interventions in aging Spinal Cord injury” (ALLRISC) aims at providing us with more knowledge on the health status and functioning of persons ageing with SCI living in the Netherlands and it will help us to formulate requirements and guidelines for a lifespan-covering rehabilitation aftercare system (34).
AcknowledgEments
This study was part of the Dutch research programme “Restoration of mobility in SCI rehabilitation: The Umbrella Project” and is financially supported by ZonMw Rehabilitation programme, grant no. 14350003 and 14350010.
The authors would like to thank the research assistants and physiatrists of the SCI units for collecting all the data, and the following participating Dutch rehabilitation centres: Rehabilitation Center De Hoogstraat (Utrecht), Reade Center for Rehabilitation (Amsterdam); Rehabilitation Center Het Roessingh (Enschede); Adelante Rehabilitation Center (Hoensbroek); Sint Maartenskliniek (Nijmegen); University Medical Center Groningen, Center for Rehabilitation-location Beatrixoord (Haren); Rehabilitation Center Heliomare (Wijk aan Zee) and Rijndam Rehabilitation Center (Rotterdam).
The authors declare no conflicts of interest.
References
