Eva Melin, MD1, Eva Lindroos, MLA2, Ingrid E. Lundberg, MD, PhD2, Kristian Borg, MD, PhD1 and Marina Korotkova, MD, PhD2,3
From the 1Division of Rehabilitation Medicine, Department of Clinical Sciences, Karolinska Institutet Danderyds Hospital, 2Rheumatology Unit, Department of Medicine, Karolinska University Hospital, Solna, Karolinska Institutet, Stockholm, 3Actar AB, Solna, Sweden
OBJECTIVE: The aim of this study was to investigate signs of inflammation in muscle of patients with prior polio, since the main symptoms in these patients are muscle pain, weakness and fatigue. In the context of pain and inflammation, the prostaglandin E2 pathway is of interest. Prostaglandin E2 has many biological actions and is a mediator of inflammation and pain.
Patients and methods: Skeletal muscle biopsies from 8 patients with prior polio and post-polio symptoms, presenting with pain and muscular weakness, and from 6 healthy controls were studied. Immunohistochemistry, conventional microscopy, and computerized image analysis were performed.
RESULTS: There was statistically significant higher expression of enzymes of the prostaglandin E2 synthetic pathway, in muscle from patients, compared with controls. Expression of prostaglandin enzymes was mainly in scattered cells and blood vessels, and may indicate an inflammatory process of the muscle, which could be secondary to systemic inflammation.
CONCLUSION: This data may indicate an inflammatory process in muscle of prior polio patients. Up-regulation of the prostaglandin E2 pathway reveals a potential background to the pain experienced by these patients, and may provide opportunities for directed pharmacological and physical therapies, which could lead to better outcomes of rehabilitation interventions.
Key words: post-polio syndrome; neuromuscular diseases; prostaglandin E2; skeletal muscle.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Eva Melin, Division of Rehabilitation Medicine, Department of Clinical Sciences, Karolinska Institutet Danderyds Hospital, Stockholm, Sweden. E-mail: eva.melin@ki.se
Accepted Jun 17, 2013; Epub ahead of print Oct 25, 2013
Introduction
Acute poliomyelitis leads to muscular weakness due to damage to the anterior horn cells of the spinal cord. After an initial recovery and a long stable period, new or increasing symptoms may arise, constituting post-polio syndrome (PPS) (1, 2). The symptoms of PPS include muscular weakness and atrophy, pain in the joints and muscles, fatigue, sensitivity to cold and sometimes dysphagia and psychological symptoms (1–3). The mechanism of development of this syndrome is not fully understood. The target of rehabilitation in PPS is to achieve a decrease in symptoms and an amelioration of function, activity and quality of life. Among the symptoms of PPS, pain may be difficult to treat and may lead to difficulty in achieving the goal of rehabilitation.
Previous studies have demonstrated that, when recovering from polio, there is a reinnervation process, leading to increased size of the remaining motor units (4, 5). It has been speculated that PPS develops when the reinnervation process is no longer capable of compensating for the denervation (4, 5). However, other hypotheses have been put forward, including immunological dysfunction, overuse of the enlarged motor units (6), an accelerating ageing process (4), persistent polio virus infection, or even a genetic predisposition (7).
A hypothesis of immunological dysfunction is supported by observations of inflammation in the cerebrospinal fluid, muscle and spinal cord in PPS patients (8–10). More recent studies, using modern techniques, have reported elevated levels of inflammatory markers, cytokines, in the cerebrospinal fluid (CSF) (7, 8, 11) and in peripheral blood (8, 12) of patients with PPS, and in a recent proteomic study significant alterations were found in CSF proteins involved in inflammation and apoptosis (7). Further support for the involvement of the immune system in PPS is the down-regulation of the inflammatory process and subsequent beneficial clinical effects with improved muscular strength, improved quality of life and decrease in pain on treatment with high-dose intravenous immunoglobulins (IvIg) (11, 13, 14).
Muscle is the major site of clinical involvement in acute poliomyelitis and PPS. Whether this is primarily due to the neurological affection or to a primary effect of PPS in the muscle tissue is not clear. There are conflicting data concerning the existence of inflammatory infiltrates in the skeletal muscle of PPS patients (10, 15, 16). In the context of pain and inflammation the prostaglandin pathway is of interest. Prostaglandin E2 (PGE2) has a large number of biological actions in the body and is a key mediator of inflammation, fever and pain and may regulate immune reactions (17, 18). PGE2 is derived from arachidonic acid via prostaglandin H2, through the action of the enzymes cyclo-oxygenase 1 (COX-1) and 2 (COX-2), and several terminal PGE2 synthases (PGES). Microsomal PGES1 (mPGES-1) is the terminal synthase involved in the inducible production of PGE2 at sites of inflammation (19). The COX-2 and the mPGES-1 are inducible enzymes up-regulated by pro-inflammatory stimuli. They generate PGE2, which typically causes inflammation including swelling, fever and pain. However, in certain conditions, mPGES-1 might act in concert with COX-1 (20–22). Two other terminal synthases, cytosolic PGES (cPGES) and mPGES-2, are constitutively expressed and are thought to produce PGE2, which is essential for physiological processes (23, 24). The PGE2 pathway is suppressed by glucocorticoids and by non-steroidal anti-inflammatory drugs (NSAIDs), both of which decrease PGE2 production (25).
PPS is clinically characterized by muscle weakness and myalgia and little is known about the pathophysiology of this condition in muscle tissue. The aim of this study was therefore to investigate signs of inflammation in skeletal muscle. Furthermore, the PGE2 pathway, a known mechanism of pain, was analysed in the muscle tissue.
Patients and methods
Patients
Eight patients with prior polio (PP) and post-polio symptoms (4 men and 4 women, mean age 39 years (standard deviation (SD) 20.6)), and 6 healthy controls (3 men and 3 women, sex- and age-matched, mean age 43 years (SD 15.4)) were included in the study (Table I). Patients were recruited consecutively from the neurological outpatient clinic of the Karolinska University Hospital in Stockholm, Sweden. All patients were referred to the outpatient clinic with muscle weakness or fatigue, and neurological and neurophysiological examinations were compatible with a lower motor neurone disorder. The healthy controls were volunteers without neurological disease. All patients had previously had poliomyelitis, diagnosed by clinical neurological and neurophysiological examinations, and had new or increasing neurological symptoms in the lower extremities, mainly muscular pain, weakness and fatigue. All patients fulfilled the criteria for PPS according to March of Dimes (2), with the exception of one patient who was 14 years old at the time of biopsy (i.e. he did not fulfil the criteria of at least a 15-year period of stable symptoms). Accordingly, the patients in this study were called PP patients. All patients were ambulant, but one used crutches. All patients had pain and 3 reported pain as their main problem. The others reported fatigue or muscular weakness as their main clinical problem. All patients had muscle weakness and weakened or loss of tendon reflexes in the lower extremities. Four patients had weakness in both legs. Muscle biopsies were performed in muscles with slight to moderate paresis and/or atrophy. None of the patients were on treatment with immunoglobulins. Subjects’ consent was obtained according to the Declaration of Helsinki, and the study was approved by the ethics review board in Stockholm.
|
Table I. Demographic data for prior polio patients and healthy controls |
||||||
|
Sex Male/female (M/F) |
Muscle biopsy VL/TA |
Age at biopsy Years |
Onset of post-polio symptoms Age, years |
Duration of post-polio symptoms Age, years |
||
|
Prior polio |
M |
VL |
29 |
25 |
4 |
|
|
Prior polio |
M |
TA |
21 |
18 |
3 |
|
|
Prior polio |
M |
TA |
65 |
60 |
5 |
|
|
Prior polio |
M |
TA |
14 |
14 |
1 |
|
|
Prior polio |
F |
VL |
44 |
38 |
6 |
|
|
Prior polio |
F |
TA |
53 |
50 |
3 |
|
|
Prior polio |
F |
TA |
65 |
51 |
14 |
|
|
Prior polio |
F |
TA |
20 |
18 |
2 |
|
|
Control |
M |
TA |
26 |
– |
– |
|
|
Control |
M |
TA |
30 |
– |
– |
|
|
Control |
M |
TA |
60 |
– |
– |
|
|
Control |
F |
TA |
37 |
– |
– |
|
|
Control |
F |
TA |
63 |
– |
– |
|
|
Control |
F |
TA |
42 |
– |
– |
|
|
VL: vastus lateralis; TA: tibialis anterior. |
||||||
Muscle biopsies
Muscle tissue samples were obtained under local anaesthesia with a semi-open muscle biopsy technique (26). Muscle biopsies were taken from polio-affected muscles, which were weak and/or atrophic, either the vastus lateralis (VL) or the tibialis anterior (TA) muscles. The biopsies were frozen and stored at –70ºC. For each biopsy, serial cryostat sections were mounted on glass slides and stored at –70ºC until stained.
Staining with immunohistochemistry
A standard immunohistochemistry protocol was applied for stainings (27), analysing for the presence of CD3-positive cells (marker for T lymphocytes), CD163-positive cells (marker of resident tissue macrophages), and CD68-positive cells (marker of monocyte/macrophage lineage), the enzymes COX-1, COX-2, mPGES-1 and cPGES. Stainings were validated using relevant positive controls (tonsil tissue, dermatomyositis or juvenile dermatomyositis skeletal muscle) and negative controls (stained with isotype-matched irrelevant antibodies) (Table II).
|
Table II. Primary antibodies used for immunohistochemical staining; the clones, species, isotypes and manufacturers |
||||
|
Name |
Primary antibody |
|||
|
Clone |
Species |
Isotype |
Company |
|
|
Anti-CD3 |
SK7 |
Mouse |
IgG1 |
BD Biosciences, San Jose, CA, USA |
|
Anti-CD68 |
EBM11 |
Mouse |
IgG1 |
DakoCytomation, Glostrup, Denmark |
|
Anti-CD163 |
Ber-MAC3 |
Mouse |
IgG1 |
DakoCytomation, Glostrup, Denmark |
|
Anti-mPGES-1 |
Polyclonal |
Rabbit |
Ig |
Westman M, 2004 (28) |
|
Anti-cPGES |
Polyclonal |
Rabbit |
Ig |
Cayman Chemicals, Ann Arbor, MI, USA |
|
Anti-COX1 |
Monoclonal |
Mouse |
IgG |
Wako Chemicals, Neuss, Germany |
|
Anti-COX2 |
Polyclonal |
Rabbit |
Ig |
Cayman Chemicals, Ann Arbor, MI, USA |
Evaluation of staining
Coded tissue sections were analysed using microscope Leica DM RXA2 (Leica Microsystems, Wetzlar, Germany), and photographed with a digital camera 300F (Leica, Cambridge, UK). For quantification, both conventional evaluation and a computerized image analysis system (Leica Qwin IM500, Leica Microsystems Digital Imaging, Cambridge, UK) were applied. Whole tissue sections were analysed.
Conventional microscopic evaluation
COX-1, COX-2, mPGES-1 and cPGES expression were first assessed qualitatively and were thereafter quantified by manual evaluation by 2 independent investigators using a semi-quantitative scale between 0 and 3, where 0 = no positive staining, 1 = very few stained cells and vessels, capillaries and larger vessels; 2 = moderate amount of positively stained cells and vessels; and 3 = strong staining in many cells and vessels. The mean value of the 2 assessments was used.
Computerized image analysis
To quantify the expression of mPGES-1, cPGES, COX-1, COX-2, CD68, CD163 and CD3 computerized image analysis was used. It was also used to assess the total tissue area of the sections. Both the percentages of the positively stained area and the number of stained cells (mainly endothelial cells of capillaries, but also a few cells in larger vessels and/or nuclei of muscle fibres) per total tissue area were analysed. To standardize the settings of the analytical condition a standard setting procedure for the microscope and computer system was applied. When necessary, part of a muscle biopsy section from a patient was used as a reference.
Statistics
The results were analysed with the IBM SPSS Statistics, version 20 programme, using non-parametric statistics, the Mann-Whitney U test. p-values < 0.05 were considered significant.
Results
Histology
In the muscle biopsies of 5 out of 8 of the PP patients’ scattered atrophic muscle fibres were observed. The remaining 3 patients had clusters of atrophic fibres. One PP patient also had a variation of muscle fibre sizes.
Inflammatory cells
There were no infiltrates with inflammatory cells in muscle biopsies of patients or controls. Scattered CD3-positive T cells and occasional CD68-positive and CD163-positive macrophages were found in all biopsies. The number of T-cells or macrophages did not differ between PP patients and healthy controls.
Expression of COX and PGES in muscle biopsies
COX-1 expression was seen in muscle biopsies of all the patients, and in biopsies of 5 of 6 of the controls (Fig. 1A, B). The expression was observed mainly in endothelial cells of capillaries and larger vessels, and a few nuclei and cytosol of scattered muscle fibres. In the PP patients, COX-1 was also expressed in scattered cells and in the cells around the vessels (Fig. 1B).
Expression of COX-2 was strong or very strong in muscle biopsies of all PP patients, and weak or absent in 5 of 6 healthy controls. COX-2 expression was mainly localized to endothelial cells of capillaries and larger vessels, but could also be seen in the cytosol of occasional muscle fibres in 5 of 8 patients and in 1 of 6 healthy controls (Fig. 1C, D). In patients, COX-2 staining was also found in a number of scattered cells and clusters of cells (Fig. 1D).
Expression of mPGES-1 was found in biopsies from all patients, and in 3 biopsies out of 6 of the healthy controls (Fig. 1E, F). mPGES-1 expression was mainly localized to capillaries and larger vessels. In patients we observed the expression of mPGES-1 in scattered cells and cell clusters (Fig. 1F), similar to COX-2 expression.
Expression of cPGES was found in muscle biopsies of all patients and in 5 of 6 healthy controls. Expression was found mainly in capillaries, larger vessels and cytosol (Fig. 1G, H). Expression of cPGES was also seen in the nuclei of muscular fibres in biopsies from 7 of 8 patients, in 1 of 6 controls, and in scattered cells in patients (Fig. 1H).
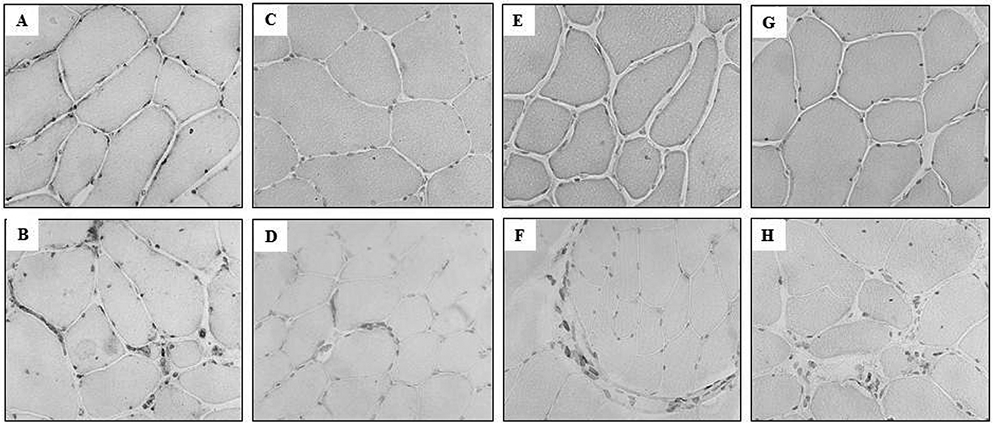
Fig. 1. Expression of COX and PGES in staining with immunohistochemistry. Immunohistochemical staining for (A) and (B): cyclooxygenase-1 (COX-1), (C) and (D): cyclooxygenase-2 (COX-2), (E) and (F): microsomal PGES 1 (mPGES-1), (G) and (H): cytosolic PGES (cPGES) in representative muscle tissue sections counterstained with haematoxylin. (A), (C), (E) and (G) from healthy individuals. (B), (D), (F) and (H) from prior polio patients (original magnification × 250).
The manual scoring and image analysis results are shown in Table III.
|
Table III. Results of manual evaluation and computerized image analysis |
|||||||||
|
Group |
Manual evaluation (0–3) |
Positively stained area 10–3 % |
Positive cells per total area (cells/mm2) |
||||||
|
Median (25–75%) |
p-value |
Median (25–75%) |
p-value |
Median (25–75%) |
p-value |
||||
|
mPGES-1 |
Healthy |
1.00 (1.00–1.25) |
0.006 |
6.95 (2.39–221.75) |
0.002 |
5.74 (2.64–114.22) |
0.002 |
||
|
Patient |
2.00 (1.63–2.50) |
102.5 (52.8–175.0) |
54.89 (35.78–102.83) |
||||||
|
cPGES |
Healthy |
1.00 (0.00–1.00) |
0.003 |
5.88 (0.34–13.21) |
0.003 |
6.01 (1.76–14.15) |
0.003 |
||
|
Patient |
2.25 (1.63–2.50) |
315.00 (180.00–432.50) |
229.71 (173.29–336.77) |
||||||
|
COX-1 |
Healthy |
2.00 (1.00–2.63) |
0.051 |
10.17 (56.75–147.50) |
0.010 |
40.91 (11.50–100.45) |
0.010 |
||
|
Patient |
2.75 (2.50–3.00) |
250.00 (147.50–500.00) |
131.41 (104.51–323.88) |
||||||
|
COX-2 |
Healthy |
0.88 (0.68–2.00) |
0.024 |
20.60 (16.18 –30.28) |
0.007 |
8.11 (5.92–13.62) |
0.007 |
||
|
Patient |
2.00 (1.63–2.88) |
65.15 (33.50–106.85) |
25.47 (18.21–57.56) |
||||||
|
CD3 |
Healthy |
– |
9.64 (3.46–29.83) |
0.156 |
1.56 (0.58–2.64) |
0.121 |
|||
|
Patient |
– |
5.10 (2.31–9.70) |
3.05 (1.23–3.40) |
||||||
|
CD68 |
Healthy |
– |
8.03 (5.23–22.83) |
0.156 |
4.40 (1.91–5.81) |
0.197 |
|||
|
Patient |
– |
3.91 (2.50–8.76) |
8.07 (3.94–12.45) |
||||||
|
CD163 |
Healthy |
– |
12.40 (7.01–23.73) |
0.106 |
6.12 (4.54–7.88) |
0.302 |
|||
|
Patient |
– |
6.59 (1.47–9.71) |
8.97 (5.23–17.54) |
||||||
Expression of COX-1 and COX-2 in the muscle tissue from the PP patients was higher than in controls. The significant up-regulation of COX-1 in PP patients was detected by manual scoring (p = 0.05) as well as by image analysis, which measured the percentage of positively stained area (p = 0.01) and the number of positive cells per total area (p = 0.01). Significant enhancement of the COX-2 staining was observed using manual scoring (p = 0.024) and image analysis, both for the positively stained area (p = 0.007) and the number of positively stained cells per total area (p = 0.007). A significantly higher expression of mPGES-1 and cPGES by manual scoring was observed in the PP patients compared with healthy controls (mPGES-1, p = 0.006, and cPGES, p = 0.003). This was confirmed by computerized image analysis. The percentage positively stained area was significantly higher in patients than in controls (mPGES-1, p = 0.002, and cPGES, p = 0.003). Likewise, the number of positively stained cells per total area was higher in PP subjects than in healthy controls (mPGES-1, p = 0.002, and cPGES, p = 0.003).
Discussion
In this study, elevated expression of the enzymes in the PGE2 pathway, mPGES-1 and COX2, along with an increase in COX-1 and cPGES expression was found in muscle biopsies from PP patients compared with healthy individuals. There were no inflammatory cell infiltrates, and only scattered inflammatory cells (T cells and macrophages) were present in the PP patients, with no significant difference compared with healthy controls. It is notable that the expression of the enzymes in the PGE2 pathway was localized primarily to scattered mononuclear cells and endothelial cells, indicating that cellular sources other than conventional inflammatory cells in infiltrates may contribute to an inflammatory environment in the skeletal muscle of the PP patients, and that PGE2 may contribute to muscle pain and muscle weakness.
Molecular changes in skeletal muscle of patients with PPS symptoms may contribute to clinical muscle symptoms, muscle weakness, pain and fatigue and they may become targets of therapy in these patients (29). Several studies have reported morphological changes in muscle of PP patients (10, 15, 16, 30–34). However, there are conflicting reports on the occurrence of inflammatory infiltrates and other signs of inflammation. In several studies inflammatory changes have been reported (10, 30, 31, 34), but were not found in others (15, 16, 32, 33). This discrepancy may be explained by differences in patient selection, or in staining techniques. However, with the use of modern immunohistochemistry technology and a sensitive microscope we could not confirm the presence of inflammatory cell infiltrates, and the scattered T-cells or macrophages were not significantly more common in PP patients than in healthy controls.
We found higher expression of enzymes in the PGE2 pathway in scattered cells, endothelial cells of micro vessels and in the muscle fibres, which could indicate an on-going inflammatory process in muscle tissue of patients with PPS symptoms. The expression of these enzymes localized to blood vessels may suggest that the inflammatory process of the muscle is secondary to a more generalized systemic inflammation. This is consistent with the findings in other studies showing an increase in inflammatory molecules such as tumour necrosis factor (TNF)-α and interleukin (IL)-6 in peripheral blood (11, 12) as well as presence of the cytokines TNF-α, interferon (IFN)-γ, IL-4 and IL-10 in cerebrospinal fluid (CSF) (8, 13, 35). An inflammatory process in PPS is further supported by the findings of alteration of CSF proteins involved in neuroinflammation, as recently revealed by proteomic techniques (7).
Pain is a frequently reported symptom in PPS patients (36). It is often localized to the muscles and of high intensity (36). However, it was shown recently that although the pain has a high intensity it does not decrease the quality of life for PPS patients, as would be expected (37). This could be due to a long-standing pain, forcing PPS patients to develop effective coping strategies. Muscle pain was present in all patients in this study and was the primary symptom in 3 of them. The mechanisms behind the up-regulation of the PGE2 pathway in muscle of the PP patients could not be determined by our study. However, it reveals a potential molecular background to the pain perceived by the patients. This could suggest that NSAIDs may be effective to reduce pain in PPS patients. Pain has been reported to correlate with TNF-α levels in peripheral blood (12). Decreased levels of TNF-α in plasma correlated with decreased muscular pain during treatment with IvIg in 1 randomized controlled study including 20 patients (13). Furthermore, IvIg treatment can down-regulate cytokine levels in CSF and can also decrease pain and increase muscle strength in patients with PPS (11, 14). Gonzalez et al. (11) speculated that the increase in muscle strength following IvIg treatment might be secondary to a decrease in pain. The present finding may increase knowledge of the background to pain in PPS and may lead to more directed and effective pharmacological and physiotherapeutic interventions, as well as to an opportunity to follow-up the interventions.
A limitation of this study is that we did not have access to peripheral blood or CSF in the present patients and therefore we could not confirm a systemic inflammation or inflammation in the CNS. Other limitations are the small sample size and that one of the patients did not fulfil all the criteria of PPS since he was 14 years old, and one criterion for PPS is a stable period of at least 15 years. However, the current patients had typical symptoms of PPS and we believe that he did not differ from a PPS patient. A strength of the study is the availability of muscle samples from healthy individuals for comparison.
In summary, in patients with PP and muscle pain there are indications of inflammation in muscle tissue through signs of activation of the PGE2 pathway. Blocking COX-1 and COX-2 are well known therapies for inflammation and pain, but these have side-effects (38, 39). The findings of this study suggest that targeting mPGES-1 might be beneficial in direct interventions for patients with PPS, leading to decreased pain and, thus, increased activity and function. However, further research is needed into this area, comparing unaffected and affected polio muscle, as well as comparisond with other neurological disorders characterized by muscle pain.
Acknowledgement
Certified Biomedical Analysist, Lisbet Broman, is acknowledged for skilful technical assistance.
Funding: This work was supported by the Swedish Rheumatism Association; and by the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet.
References
