Astrid C. J. Balemans, MSc1,2, Leontien van Wely, MSc1,2, Anouk Middelweerd, MSc2, Josien C. van den Noort, PhD1, Jules G. Becher, MD, PhD1,2 and Annet J. Dallmeijer, PhD1,2
From the 1Department of Rehabilitation Medicine, MOVE Research Institute Amsterdam, VU University Medical Center and 2The EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands
OBJECTIVE: To compare daily stride rate activity, daily exercise intensity, and heart rate intensity of stride rate in children with cerebral palsy with that of typically developing children.
METHODS: Forty-three children with cerebral palsy, walking without (Gross Motor Function Classification System (GMFCS) I and II) or with (GMFCS III) an aid and 27 typically developing children (age range 7–14 years) wore a StepWatchTM activity monitor and a heart rate monitor. Time spent and mean heart rate reserve at each stride rate activity level and time spent in each mean heart rate reserve zone was compared.
RESULTS: Daily stride rate activity was lower in children with cerebral palsy (39%, 49% and 79% in GMFCS I, II and III, respectively) compared with typically developing children (p < 0.05), while there were no differences in time spent at different mean heart rate reserve zones. Mean heart rate reserve at all stride rate activity levels was not different between typically developing children, GMFCS I and II, while mean heart rate reserve was higher for GFMCS III at stride rates < 30 strides/min (p < 0.05).
CONCLUSION: Stride rate activity levels reflect the effort of walking, in children with cerebral palsy who are walking without aids, similar to that of typically developing children, whereas children with cerebral palsy using walking aids show higher effort of walking. Despite a lower stride rate activity in cerebral palsy, daily exercise intensity seems comparable, indicating that the StepWatchTM monitor and the heart rate monitor measure different aspects of physical activity.
Key words: walking; exercise; heart rate; monitoring; ambulatory; cerebral palsy.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Astrid C. J. Balemans, VU University Medical Centre, Department of Rehabilitation Medicine, MOVE Research Institute, PO Box 7057, 1007 MB Amsterdam, The Netherlands. E-mail: a.balemans@vumc.nl
Accepted Jul 3, 2013; Epub ahead of print Nov 6, 2013
Introduction
According to the World Health Organization (WHO), maintaining a physically active lifestyle during childhood is important for the development of “healthy musculoskeletal tissues, a healthy cardiovascular system, neuromuscular awareness, and to maintain a healthy body weight” (1). Persons with a physical disability are at greater risk for developing co-morbidity during adulthood, such as cardiovascular diseases, diabetes, obesity and osteoporosis as a result of reduced physical activity (2). As physical activity levels during adulthood correspond to the level of physical activity during childhood, it is especially important that children with a disability should have adequate levels of physical activity (3).
Cerebral palsy (CP) is the most common cause of physical disability in children and is defined as “a group of disorders of the development of movement and posture causing activity limitations that are attributed to non-progressive disturbances that occurred in the developing foetal or infant brain” (4, 5). These children have difficulty in performing daily activities due to their motor impairments, which includes spasticity, impaired selective motor control and increased co-activation (5). The mobility level of children with CP is classified according to the Gross Motor Function Classification System (GMFCS). This system identifies 5 levels, with children classified as GMFCS level I (walking without restriction) and II (walking with restrictions) able to walk without walking aids, and those classified as GMFCS level III able to walk with walking aids (6). These limitations in performing daily activities may cause reduced levels of daily physical activity (7–9).
Methods for monitoring physical activity have been developed (10) in order to detect persons with and without a disability who are at risk of developing an inactive lifestyle (11). Objective measurements of physical activity range from sophisticated methods that estimate total energy expenditure (EE), such as by using doubly labelled water (9), to child-friendly approaches, such as the accelerometry-based StepWatchTM activity monitor, which estimates the total number of strides taken and the time spent at different stride rates per day (2, 12). Methods for monitoring physical activity may provide information on the total amount of physical activity or on the patterns of physical activity performance; duration, frequency, intensity and mode of activity (13).
Previous studies found that children with CP expend less energy during the day compared with children who develop typically (TD), as measured with doubly labelled water (9, 14) or estimated based on the relationship of heart rate (HR) to oxygen uptake (VO2) (15). Unlike the small studies that estimated daily EE, studies measuring stride rate were able to include larger samples (12). These studies found that children with CP take fewer strides per day compared with TD (7), and that the number of strides deteriorates as the level of motor involvement increases (16).
The development of guidelines for physical activity is aimed mainly at healthy individuals and based on the amount of energy expended during the day (17). However, children with CP have a higher energy cost of walking, which increases with increasing motor involvement (18). It is therefore doubtful that comparison of stride rate to TD is an appropriate method for determining intensity of physical activity in children with CP. The exercise intensity of stride rate activity provides information on the effort of walking in daily life. A method that can be used to express the exercise intensity of activities is the heart rate reserve (HRR). HRR indicates the relative stress placed on the cardiorespiratory system and reflects the rate of EE during physical activity (19). The HRR can therefore be used to estimate the effort of all daily physical activity, including activities other than walking, and to estimate the intensity of stride rate activity levels. The present study was performed to obtain insight into whether activity monitors, such as StepWatch, are suitable in CP when the aim is to estimate intensity of physical activity. The aim of this study was to compare daily stride rate activity and daily exercise intensity measured by heart rate, and the heart rate intensity of different stride rate activity levels in walking children with CP and in TD children.
Methods
Participants
Walking children with spastic CP (GMFCS I-III), aged between 7 and 14 years, were recruited through paediatric physiotherapy practices and special schools for children with physical disabilities. All children with CP participated in a trial to evaluate a physical activity stimulation programme, which included fitness training and physical activity stimulation through counselling and home-based physical therapy (20). TD were matched on age and gender and were recruited from elementary schools or through colleagues at the department. Before enrolment, the following exclusion criteria were checked: (i) contra-indications for maximal exercise, and for the CP participants; (ii) botulinum toxin treatment or serial casting less than 3 months previously; and (iii) surgery less than 6 months previously. The study was approved by the institutional medical ethics board, and participants over 12 years of age and all parents signed an informed consent form.
Procedure
Children visited the outpatient clinic of the hospital for anthropometric measurements, to perform the exercise tests, and to calibrate the StepWatch. In order to calculate HRR, peak HR was determined in an incremental aerobic exercise test until exhaustion on a cycle ergometer (21). After the measurement, the children were asked to wear the StepWatch for 7 consecutive days, enabling reliably measuring step activity (22), during waking hours, with the exception of bathing time or swimming. Simultaneously with the StepWatch, children wore a HR monitor for 3 days (2 week days, 1 weekend day), day and night. Children and/or parents were asked to keep an activity diary, in which times of getting up, going to sleep and periods of not wearing the StepWatch were registered.
Equipment
Walking activity was measured with the ankle-worn biaxial StepWatchTM Activity Monitor 3.0 (Orthocare Innovations, Seattle, WA, USA) that registers accelerations of one leg in the frontal–sagittal plane per time interval (23). The StepWatch device was calibrated while the subject walked on an oval 50 m track, with strides counted manually and concurrently recorded with the StepWatch device. Sensitivity settings of the StepWatch device were adjusted until manual counting and StepWatch recordings agreed > 95%. An agreement of > 95% between manual counting and StepWatch counting was not established for both walking and running with one sensitivity setting; therefore, we adjusted the sensitivity setting for walking. HR was measured with a HR monitor (RS400, Polar Electro, Kempele, Finland). Mean values were stored every minute (for the StepWatch) and every 5 s (for the HR monitor), providing an outcome measure of mean strides/min and mean beats/min. The internal clocks of the StepWatch and the HR monitor were synchronized. The maximal exercise test was performed on a cycle ergometer (Corival V2; Lode BV, Groningen, the Netherlands) with a HR monitor (Cosmed S.r.l.) storing every HR beat.
Data analysis
StepWatch and HR data were analysed using MATLAB Software (Version 7.12.0.635, R2011a, The Mathworks). Days were excluded from the analysis if: (i) > 3 h data were missing within the time interval being awake; and (ii) a day had < 10 h (week day) or < 8 h (weekend day) of registration time.
Daily stride rate activity and heart rate response. Time spent at different stride rate activity levels was calculated over the wearing time interval. The different stride rate activity levels were based on previously published cut-off points (16, 24, 25): 0 strides/min (SR0), 1–15 strides/min (SR1–15), 16–30 strides/min (SR16–30), 31–60 strides/min (SR31–60) and > 60 strides/min (SR > 60).
In addition, time spent in different HRR zones was calculated. The HRR zones were based on American College of Sports Medicine (ACSM) guidelines for physical activity intensity (17): very light (< 30%); light (30–40%); moderate (40–60%); vigorous (60–90%); and near-maximal to maximal (≥ 90%). Time spent at the different HRR zones was calculated in minutes and as a percentage of the daily registered time. HRR was calculated with the following formula: [100% × (HRstride–HRrest)/(HRpeak–HRrest))]. HRrest was determined as the lowest HR measured during sleep. HRpeak was determined as the highest registered HR achieved during the maximal aerobic exercise test, or during the daily HR registration if a higher value was achieved. Intensity over a whole day was also expressed as time spent in HR zones: very light (< 100 beats/min); light (100–115 beats/min); moderate (115–140 beats/min); vigorous (140–180 beats/min); and near-maximal to maximal (≥ 180 beats/min). In addition, the percentage of children per group that met the physical activity guideline was calculated: spending at least 60 min at moderate (> 40% HRR) intensity, of which 30 min at vigorous (> 60% HRR) intensity (26). Time spent in sustained periods of longer than 5, 10 and 20 min at HRR > 60% was also calculated.
Intensity of stride rate activity levels. Mean HRR and mean HR at each stride rate activity level was calculated for the daily registration period in order to determine the intensity of the previously defined stride rate activity levels for each group. For this part the HR data were averaged over 1 min epochs, enabling the data to be synchronized to the StepWatch data. The HR data was synchronized to the StepWatch data using the starting date and time of the registration period.
Statistical analysis
Sample size calculation showed that at least 12 children were required in each GMFCS group to detect a difference in HRR of 10% between groups (TD, GMFCS I, II or III) with a power of 0.8, an alpha of 0.05 and a standard deviation (SD) of 9% HRR. Statistical analysis was performed using Predictive Analytics SoftWare (PASW) Statistics 18 (SPSS Inc., Chicago, IL, USA). Depending on the distribution of the data, parametric or non-parametric analyses were performed. Data were checked for confounding by height and gender. Data were presented as the mean (SD) when data were normally distributed, otherwise as the median with the interquartile range. Data were analysed using a one-way analysis of variance (ANOVA) using post-hoc Bonferroni adjustments or a Kruskal-Wallis test, and a p-value of < 0.05 was considered significant.
Results
Participants
A total of 70 children participated in this study (age range 7–14 years old); 43 children with CP (GMFCS I-III) and 27 TD. Specific characteristics are detailed in Table I and show no significant differences between groups. The HR registration days of 3 TD and 3 children with CP (GMFCS I: n = 1, II: n = 1, III: n = 1) did not meet the predefined inclusion criteria for a total registration day, due to data recording failure. One child (female, age 9 years 1 month, GMFCS III, bilateral involved) was observed to be an outlier, with HRR values > 5 SD from group means. This child was subsequently eliminated from the HRR analysis, without any influence on the conclusions. Finally, 70 children remained in the analysis for time spent at stride rate activity levels and mean HRR at each stride rate activity level, while 63 children remained in the analysis for time spent at HRR zones. Analysis showed that confounding for height and/or gender was not present in the data.
|
Table I. Characteristics of the participants |
||||
|
|
TD (n = 27) |
Children with CP (n = 43) |
||
|
GMFCS I (n = 23) |
GMFCS II (n = 12) |
GMFCS III (n = 8) |
||
|
Boys/girls, n |
11/16 |
15/8 |
6/6 |
4/4 |
|
Age, years, mean (SD) |
10.1 (1.5) |
10.5 (2.1) |
9.5 (1.1) |
9.4 (1.3) |
|
Height, cm, mean (SD) |
144 (11.5) |
143 (14.1) |
137 (10.8) |
132 (5.6) |
|
Weight, kg, mean (SD) |
37.0 (7.3) |
37.7 (13.2) |
35.1 (11.5) |
34.3 (9.9) |
|
Unilateral/bilateral, n |
NA |
18/5 |
4/8 |
0/8 |
|
HRrest, beats/min, mean (SD) |
55 (7.1) |
57 (6.9) |
57 (8.3) |
63 (9.9) |
|
HRpeak, beats/min, mean (SD) |
197 (9.7) |
200 (13.6) |
200 (15.1) |
190 (9.6) |
|
CP: cerebral palsy; SD: standard deviation; GMFCS: Gross Motor Function Classification System; TD: typically developing children; NA: not applicable. |
||||
Daily stride rate activity and heart rate response
Table II shows time spent (in min) at each stride rate activity level. During a day, children with GMFCS II and III spent more time at SR0 (+11% (NS), +31%, +40% GMFCS I, II, III, respectively) and less time at SR1–15 (–6% (NS), –17%, –18% GMFCS I, II, III, respectively). All children with CP spent less time at SR16–30 (–24%, –29%, –32% GMFCS I, II, III, respectively) and SR31–60 (–39%, –49%, –79% GMFCS I, II, III, respectively) than TD (p < 0.001). No difference was found for time spent in SR > 60 between groups (Table II, Fig. 1).
|
Table II. Time (min) spent in stride rate activity levels and mean heart rate at different stride rate activity levels |
||||||||
|
TD (n = 27) Mean (SD) |
Children with CP (n = 43) |
One-way ANOVA |
||||||
|
GMFCS I (n = 23) Mean (SD) |
GMFCS II (n = 12) Mean (SD) |
GMFCS III (n = 8) Mean (SD) |
F |
p-value |
Post-hoc |
|||
|
SR0 |
||||||||
|
Time, min |
286 (58.9) |
317 (75.1) |
375 (94.1) |
400 (81.7) |
7.297 |
0.000* |
TD-III, TD-II, I–III |
|
|
HR, beats/min |
92 (8.4) |
95 (10.1) |
95 (6.2) |
106 (9.6) |
4.811 |
0.004* |
TD-III, I–III |
|
|
SR1-15 |
||||||||
|
Time, min |
284 (36.4) |
268 (49.4) |
236 (35.0) |
234 (29.0) |
5.692 |
0.002* |
TD-II, TD-III |
|
|
HR, beats/min |
99 (7.9) |
101 (10.7) |
102 (4.8) |
111 (8.7) |
4.271 |
0.008* |
TD-III, I–III |
|
|
SR16–30 |
||||||||
|
Time, min |
110 (21.4) |
84 (20.8) |
78 (23.0) |
35 (14.0) |
28.209 |
0.000* |
TD-I, TD-II, TD-III, I–III, II–III |
|
|
HR, beats/min |
110 (8.1) |
109 (11.7) |
111 (7.2) |
119 (8.7) |
2.489 |
0.068 |
– |
|
|
SR31–60 |
||||||||
|
Time, min |
100 (34.4) |
61 (22.7) |
51 (24.4) |
20.5 (21.1) |
21.541 |
0.000* |
TD-I, TD-II, TD-III, I–III |
|
|
HR, beats/min |
120 (9.4) |
120 (15.4) |
118 (6.4) |
124 (16.6) |
0.390 |
0.761 |
– |
|
|
SR > 60 |
||||||||
|
Time, min |
5 (1–11.5) |
3.5 (1.5–9.6) |
4.5 (1.08–6.75) |
0 (0–12.25) |
0.617 |
0.554a |
– |
|
|
HR, beats/min |
121 (11.2)b |
131 (22.6)c |
129 (18.1)d |
NA |
1.076 |
0.368 |
– |
|
|
*Indicates significance. aNon-parametric Kruskal-Wallis test, data presented as median (interquartile range). b (n = 19). c (n = 18). d (n = 10). SD: standard deviation; HR: heart rate; TD: typically developing children; GMFCS: Gross Motor Function Classification System; NA: not applicable; SR0: 0 strides/min; SR1–15: 1–15 strides/min; SR31–60: 31–60 strides/min; SR > 60: > 60 strides/min; 3 out of 8 children achieved SR > 60. |
||||||||
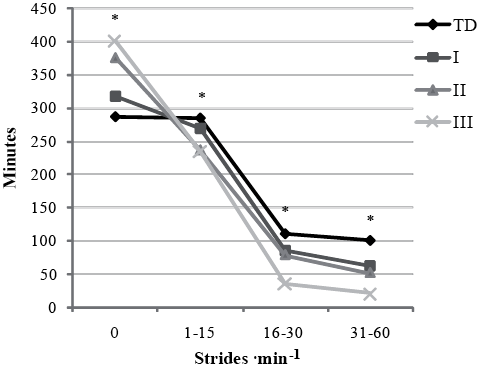
Fig. 1. Time (min) spent in stride rate activity levels. *p < 0.05; standard deviation (SD) not shown. SR > 60 not shown since the data had no normal distribution. TD: typically developing children; GMFCS: Gross Motor Function Classification System.
The time spent in each HRR zone during the day did not significantly differ between children with CP in all GMFCS levels and TD (Table III, Fig. 2). Time spent at different HR zones also did not differ between children with CP in all GMFCS levels and TD (data not shown). The percentage of children that met the physical activity guideline of > 60 min at HRR > 40%, of which > 30 min at HRR > 60% were as follows: TD: 58%; GMFCS I: 59%; GMFCS II: 46%; GMFCS III: 57%. All groups achieved a comparable amount of sustained time (> 5, > 10, > 15 min) above HRR 60%. In the case of > 5 min, medians (interquartile range) were: TD: 13.5 (6.6–22.1); GMFCS I: 12.8 (5.9–28.7); GMFCS II: 6.7 (0–18); and GMFCS III: 9 (0–15) (p < 0.449).
|
Table III. Time (min) spent in heart rate reserve (HRR) zones per day |
|||||||
|
TD (n = 24) Mean (SD) |
Children with CP (n = 39) |
One-way ANOVA |
|||||
|
GMFCS I (n = 22) Mean (SD) |
GMFCS II (n = 11) Mean (SD) |
GMFCS III (n = 6) Mean (SD) |
F |
p-value |
|||
|
Very light (< 30%), time, min |
360.2 (115.5) |
353.7 (136.0) |
320.8 (127.1) |
242.9 (122.8) |
1.571 |
0.206 |
|
|
Light (30–40%), time, min |
184.0 (87.1) |
161.3 (70.2) |
186.3 (75.4) |
199.0 (58.2) |
0.646 |
0.588 |
|
|
Moderate (40–60%), time, min |
128.7 (44.0) |
118.1 (71.8) |
113.5 (68.9) |
144.2 (43.8) |
0.465 |
0.708 |
|
|
Vigorous (60–90%), time, min |
37.3 (23.6) |
44.4 (26.7) |
29.1 (25.9) |
39.5 (22.8) |
0.946 |
0.424 |
|
|
Near-max to maximal (≥ 90%), time, min |
2 (0–4) |
2 (0–3.5) |
1.5 (0–2.5) |
1 (0.75–1.88) |
2.030a |
0.566a |
|
|
aNon-parametric Kruskal-Wallis test, data presented as median (interquartile range). GMFCS: Gross Motor Function Classification System; SD: standard deviation; interquartile range; TD: typically developing children. |
|||||||
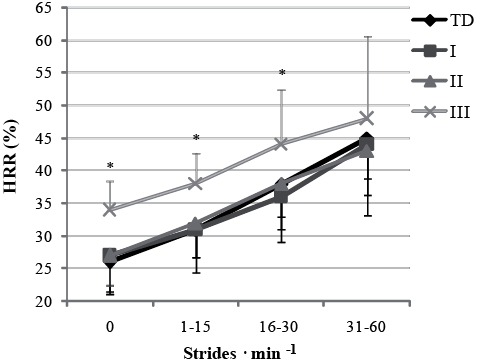
Fig. 2. Mean (standard deviation; SD) heart rate reserve (HRR) in stride rate activity levels, SR>60 not shown since the data had no normal distribution. TD: typically developing children; GMFCS: Gross Motor Function Classification System. *p < 0.05.
Intensity of stride rate activity levels
At all stride rate activity levels, mean HRR and mean HR were not different between TD, GMFCS I and II (Table II). Mean HRR was higher for children in GFMCS III compared with TD, GMFCS I and GMFCS II at SR0 and SR1–15, and mean HRR was higher for children in GMFCS III compared with GMFCS I at SR16–30 (p < 0.05) (Fig. 3).
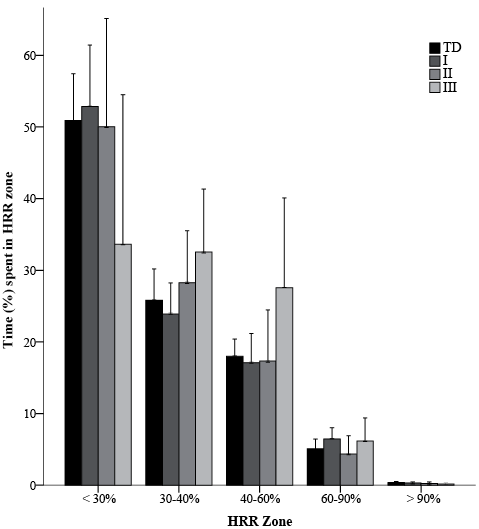
Fig. 3. Time (mean (95% confidence interval)) spent at heart rate reserve (HRR) zones as a percentage of total time. TD: typically developing children; HRR: heart rate reserve; GMFCS: Gross Motor Function Classification System; *p < 0.05.
Discussion
The objective of this study was to compare daily stride rate activity and HRR, and the heart rate intensity of different stride rate activity levels in walking children with CP and with TD children. The results show that the HRR intensity of stride rate activity levels is at a similar level in TD and GMFCS I and II, but is higher in GMFCS III. Although stride rate activity was lower for children with CP compared with TD, children with CP seem to spend a comparable amount of time in the different HRR zones during the day to that of TD children.
To the best of our knowledge, the HRR intensity of stride rate activity levels has never been determined in children with CP. Our results show that the effort of walking is well determined by the use of stride rate activity levels in children with CP, classified as GMFCS I and II, when compared with TD. The effort of walking therefore seems to be comparable with that of TD children. Nevertheless, children with CP in all GMFCS levels spent less time in higher stride rate activity levels and more time at SR0, which confirms that walking activity is reduced in children with CP (7, 27). Taking into consideration the fact that children with CP show a higher EE when walking 1 m (energy cost), our results indicate that EE per stride is similar between TD and GMFCS level I and II (18). It is likely that GMFCS I and II have a smaller stride length, perhaps due to impaired coordination, spasticity and disturbed balance, and consequently cover a smaller distance (28). Therefore, stride rate appears to be an appropriate measure for monitoring the intensity of walking activity in children with CP who walk without walking aids, compared with TD.
Children with CP classified as GMFCS level III showed higher levels of HRR intensity at most stride rate activity levels than those classified as GMFCS level I and II and TD. Apparently, these children have a higher EE per stride than children who walk without walking aids, probably as a result of more severe motor impairments (5, 18). The higher HRR intensity found at SR0 might be due to a higher intensity during sitting and standing, probably attributable to greater difficulty in stabilizing the trunk and controlling balance (5). Children with higher GMFCS levels and involvement of the upper extremities also show a higher EE when performing activities while sitting, when using their arms or when using their wheelchair over long distances (6, 29). The effort of walking is underestimated when determining physical activity by comparing stride rate activity with TD. Future research should focus on activity monitoring for GMFCS III which includes wheelchair use.
Although the intensity of stride rate activity levels is comparable between children with CP (GMFCS I and II) and TD children, children with CP have a lower stride rate activity than TD children. This suggests a lower exercise intensity during the day. However, the time spent at HRR zones was comparable for these groups. This discrepancy might result from the different activities that are captured by these measurement instruments. A HR monitor also captures activities other than walking and running, while the ankle-worn step rate monitor only captures walking activity. Previous studies have determined physical activity in CP and TD by measuring total EE over the day, using doubly labelled water or individual HR-VO2 relationships, and have shown that children with CP expend less energy compared with their peers (9, 14, 15). Since children with CP may have a different HR-VO2 relationship than TD, with a lower amount of oxygen uptake per heart beat increase as a result of a lower maximal oxygen uptake (21), this might explain our finding of comparable HRR intensities over the day, with a lower total EE in comparison with TD children (9, 14, 15).
The number of children with CP who met the physical activity guideline, based on HRR intensity, was comparable to TD (spending at least 60 min at moderate intensity, of which at least 30 min at vigorous intensity) (26). Although these guidelines were mainly developed for healthy children, the ACSM prescribes that mildly involved individuals with cerebral palsy should follow the aerobic exercise training guidelines for the general population (26). However, it is questionable whether training guidelines for healthy individuals are suitable for children with a disability; further research is therefore required.
There are various approaches to prescribing physical activity and to defining exercise intensity. Discussions involve how many strides per day are sufficient, the amount of energy that should be expended, or how many minutes should be spent in exercise intensity zones based on either %VO2 reserve, %HRmax or %HRR (12). We have defined exercise intensity with HRR zones based on those reported in the ACSM guidelines, which are mainly aimed at healthy individuals (17). HRR is preferred when measuring exercise intensity of fitness-inducing activities. When interpreting stride rate activity, there is no consensus on cut-off points for establishing moderate or vigorous intensity of physical activity. At stride rate > 60 strides/min HRR was still lower than 55%, whereas < 60% HRR is still considered moderate exercise intensity according to ACSM guidelines (17). An explanation for the relatively low HRR at the highest detected stride rates could be that, as the StepWatch was calibrated for walking it measured running less accurately, as was noticed during calibration, and this conclusion supported by the low number of children in all groups achieving this stride rate (30). Furthermore, while the StepWatch averages over 1 min intervals, short bursts of running might be shorter. Therefore, it appears that stride rate activity should preferably be used for monitoring daily walking activity, necessary for developing the musculoskeletal system and enabling participation in daily life, while HRR intensity is better suited to capturing the vigorous intensity exercise necessary for improving or maintaining physical fitness (31).
In this study, we determined mean HRR intensity at different stride rate activity levels. Both measurement instruments are known to have limitations in the determination of physical activity: StepWatch outcomes can be biased by children not wearing the StepWatch and has not been validated for activities such as cycling, an important physical activity in the Netherlands. However, the cycling motion in the vertical plane is most likely to be captured by the StepWatch, which measures accelerations in the vertical plane. HR can be influenced by other factors, such as emotional status. However, the similar mean HRR increase with increasing stride rate activity levels in all groups suggests that these factors did not influence our conclusions (Fig. 1). A limitation is the small number of children classified as GMFCS II and III that participated in the study, therefore, the results should be confirmed in future research with a larger sample. Time spent at different HR zones was also not different between children with CP in all GMFCS levels and TD, indicating that differences in HRsleep and/or HRpeak are not responsible for the differences in HRR between groups.
In conclusion, stride rate activity levels reflect the effort in walking activity in children with CP walking without aids similar to that of TD, whereas children with CP who use a walking aid show a higher HRR intensity at lower stride rate levels, indicating a higher effort of walking. Despite a lower stride rate activity for children with CP, daily exercise intensity seemed to be comparable between groups. These findings indicate that the StepWatchTM monitor and the heart rate monitor measure different aspects of physical activity; stride rate activity might preferably be used for monitoring daily walking activity, while HRR intensity may be better suited to capturing vigorous intensity exercise.
ACKNOWLEDGEMENTS
We would like to thank all children and their parents for participating in this study. This study was supported with a grant from “The Netherlands Organisation for Health Research and Development (ZonMw)” and the “Phelps Foundation for Spastics”.
References
