Sheng Li, MD, PhD1,2, Ana Durand-Sanchez, MD3 and Mark L. Latash, PhD4
From the 1Department of Physical Medicine and Rehabilitation, University of Texas Health Science Center at Houston, 2UTHealth Neurorehabilitation Research Laboratory at TIRR, The Institute of Rehabilitation and Research (TIRR) new primary melanomaMemorial Hermann Hospital, Houston, TX, 3Department of Physical Medicine and Rehabilitation, Indiana University Medical School, Indianapolis, IN and 4Motor Control Laboratory, Department of Kinesiology, Pennsylvania State new primary melanomaUniversity, State College, PA, USA
OBJECTIVE: Interlimb coupling between impaired and non-impaired limbs after stroke has been a common observation. The aim of this study was to examine interlimb interactions in force production in responses to altered visual gain in hemiparetic stroke survivors.
DESIGN: prospective clinical study
METHODS: A convenient sample of 7 hemiparetic stroke subjects (3 women and 4 men; mean age 56.0 years (standard errors 12.8) of age; history of stroke: mean duration 61.6 months (standard errors 53.3)) participated in the study. Subjects performed bilateral elbow flexion to varying total force targets from 3% to 60% maximal contraction forces with normal visual gain (1:1) and to a 10% maximal voluntary contraction target with altered visual gains (1/8, 1/4, 1/2, 2, 4, and 8) for the force of the less-impaired, ipsilesional side.
RESULTS: Across all conditions, the forces produced by both impaired and non-impaired limb changed proportionally to their maximal voluntary contraction force, such that relative contributions of each limb’s force to the total force remained unchanged. In conditions with altered visual gain, high and low, the total force showed errors in the direction of undershooting.
CONCLUSION: Our findings indicate that there is a strong interlimb force coupling in hemiparetic stroke, resistant to distorted visual feedback. It may reflect a default sharing pattern dominant after stroke.
Key words: stroke; interlimb coupling; force; rehabilitation; interlimb interaction.
J Rehabil Med 2014; 46: 206–211
Correspondence address: Sheng Li, MD, PhD, UTHealth Neurorehabilitation Research Laboratory at TIRR, TIRR-Memorial Hermann Hospital, 1333 Moursund, Houston TX 77030, USA. E-mail: sheng.li@uth.tmc.edu
Accepted Sep 17, 2013; Epub ahead of print Feb 13, 2014
Introduction
Stroke survivors often have weakness on the impaired (contralesional) side accompanied by impaired voluntary force control (1, 2). Altering visual gain has been integrated into robotic rehabilitation programs to improve motor recovery for the impaired side, particularly in unilateral reaching movements (3, 4). If force production of the impaired side could be selectively changed via adjustments of visual gains, as it has been demonstrated in healthy subjects (5, 6), altered visual gain may be integrated into bilateral training programs to improve strength on the impaired side.
Strength asymmetry after stroke may be expected to influence inter-limb interactions when both the impaired and non-impaired sides produce isometric forces simultaneously in bilateral tasks when subjects are explicitly instructed to produce a certain magnitude of the total force to match a target. Without specific instructions on force production on each side, healthy subjects have relatively equal force contributions to the total force from both sides (7), while hemiparetic stroke subjects produce less force on the impaired side (7, 8); the sharing of the total force between the two sides is consistent over a broad range of total force magnitudes (5% to 65% of maximal strength). When stroke subjects are explicitly instructed to produce equal magnitudes of force simultaneously on the impaired and non-impaired sides without an explicit target, there is a fairly constant ratio of the two forces regardless of sensory impairment on the impaired side (9). This ratio is close to the ratio of maximal voluntary contraction forces (MVCs) of the two sides (9). These reports collectively suggest a strong interlimb coupling of neural commands during isometric force production. Similarly, exaggerated interlimb coupling has also been observed in other activities in hemiplegic stroke, such as reaching (10), pedaling (11), circle drawing (12), finger tapping (13), and arm oscillation (14).
It has been recently shown that, when healthy subjects perform accurate force production tasks with visual feedback computed using altered gains of individual forces, changes in individual forces and their ratios are observed that correlate with the gain values (5, 6). In the present study, we used changes in the visual gain during bilateral isometric force production tasks in chronic hemiparetic stroke survivors. We hypothesized that altering visual gains for the force produced by one limb would change force output of that limb, and also the force produced by the contralateral limb via inter-limb interactions. In particular, the non-impaired side is expected to produce greater force when it is made “weaker” (via smaller gain), while the impaired limb is predicted to increase its force output to keep the force ratio between the two limbs constant.
Methods
Subjects
A convenient sample of 7 hemiparetic stroke subjects (3 females and 4 males; mean age 56.0 years (standard errors; SE 12.8) ; time after stroke: mean duration 61.6 months (SE 53.3), ranging from 32 to 180 months) were recruited. Inclusion criteria were: 1) hemiplegia secondary to a single stroke (hemorrhagic or ischemic) defined based on subject’s history without an additional magnetic resonance imaging (MRI); 2) at least 12 months post-stroke; 3) ability to generate elbow flexion against gravity on the impaired side; 4) a full range of passive motion in the impaired shoulder and elbow joints; 5) ability to understand instructions related to the experiments and give informed consent. Exclusion criteria were: 1) history of multiple strokes or bilateral involvement; 2) contracture or significant spasticity that would limit passive motion on the impaired side; 3) visual and/or spatial neglect interfering with using visual feedback in the study (see later); 4) cognitive deficit that did not allow subjects to follow commands. All subjects gave informed consent prior to participation. This study was approved by the local ethics committee.
Procedure
Apparatus: we adopted our previous experimental setting (1). Subjects were seated on a height-adjustable chair. Both upper limbs were symmetrically positioned with shoulders slightly flexed and abducted to approximately 45°, elbows flexed to approximately 90°, and forearms/wrists were in a neutral position. Load cells (208C02; PCB Piezotronics, Depew, NY) were placed perpendicular to the distal end of each forearm to measure the isometric elbow flexion force. Shoulder straps were used to hold the trunk against a firm back support. Extra stabilization straps were applied to the distal forearm on both sides. Force signals were digitized at 1000 Hz (PCI-6229, National Instruments, Austin, TX) using a personal computer with custom LabVIEW software (National Instruments) and saved for off-line analysis.
Tasks: Subjects first performed a series of 3 maximum elbow flexion attempts with two limbs simultaneously without visual feedback. The highest value of flexion force across the 3 trials was selected as the bilateral elbow flexion MVC (MVCBI). MVCBI was then used to create visual targets. Subjects were then instructed to perform two sets of tasks: with and without visual gain alteration. In one set of tasks, a visual target was presented corresponding to 3%, 6%, 10%, 20%, 30%, 40%, 50% or 60% of MVCBI with a thick red horizontal line on the computer screen. The screen was about 1 m in front of the subject. A real-time visual feedback of the total force (FFB) ran from left to right across the screen. FFB was the sum of the impaired limb force (FP) and the non-impaired limb force (FNP): FFB = FP + FNP. Both visual gains were set at unity so that FFB corresponded to the actual total force.
In another set of tasks with the target level at 10%MVCBI, the gain of the visual display of FNP was altered, while the visual gain of FP was not changed. As such, FFB was a weighted sum of the forces from two limbs, FFB = FP + FN × G. The G values were 1/8, 1/4, 1/2, 2, 4, and 8. Subjects were aware of the gain change, but not of the specific gain. Subjects practiced performing elbow flexion on each side at each visual gain value (3–4 trials), but not the two-arm force production.
For all tasks, each trial lasted 11 s. Subjects were given auditory cues to start bilateral elbow flexion 3 s after and to relax 9 s after the beginning. Subjects were explicitly instructed to match the target line with FFB while producing force simultaneously by both limbs. The same instructions were given for all tasks with and without visual gain changes. Throughout the experiment, subjects were reminded to refrain from moving their trunk, shoulders, elbows, or wrists. Three trials were performed at each task with at least 30-s rest periods to minimize the fatigue effect. The two sets of tasks were presented in a balanced order while the order of conditions (G values) was randomized across subjects.
Data analysis
Force signals were analyzed offline using a custom MATLAB program. Individual elbow flexion forces were averaged over a 2-s window between 6–8 s to standardize the analysis across tasks and trials. These values were absolute values of FNP and FP. FNP and FP were then normalized to individual MVCBI values to obtain relative values of FNP and FP. To examine whether there were any changes in the forces between limb forces after visual gain manipulations, the following analyses were performed: 1) the ratio of absolute FNP and FP was calculated in all bilateral tasks; 2) linear regression analysis was performed between absolute FNP and FP in tasks with and without visual gain manipulations, respectively. Matching performance was quantified by matching error. Matching error was defined as the difference between FFB and the target force with and without visual gain manipulation. A positive value indicates an overshooting, while a negative value means an undershooting. Visual gain manipulations led to change in the actual total force for the same 10%MVC visual target. To compare match errors at different actual total force with and without visual gain manipulation, non-linear regression analysis with best curve fitting for the force-matching error relation was performed.
Statistical analysis
Descriptive statistics and repeated-measures one-way analysis of variance were used. Paired t-tests were used to compare the force ratio values. A one-way ANOVA was performed on matching error with a factor of GAIN (7 levels) for tasks with altered visual feedback or FORCE-LEVEL (8 levels) for tasks with unchanged visual feedback. Two-way ANOVAs with factors of GAIN and SIDE (2 levels) were used to examine their effect on force expressed in % on MVCBI. Tukey’s HSD tests were performed to explore significant effects in ANOVA. The alpha level was set at 0.05. Data are reported as means ± standard errors (SEs) in both the text and figures.
Results
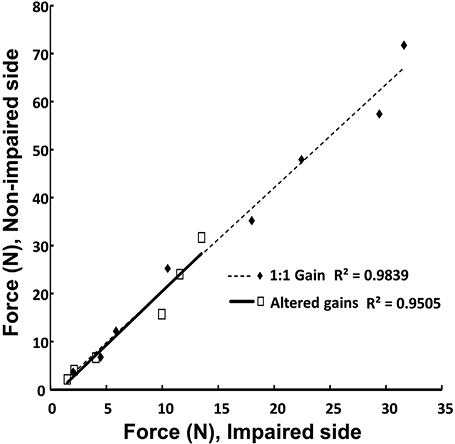
As expected, subjects produced more force on the non-impaired side (mean ± SE 123.2 ± 18.0 N) than on the impaired side (56.1 ± 7.6 N) during bilateral elbow flexion MVC tasks. The mean force ratio between MVCNP and MVCP, was 2.20. During bilateral voluntary elbow flexion at submaximal levels (3%–60% MVC) with unchanged visual gain, the ratio of FNP/FP was consistent throughout the tested force range. The mean ratio (2.44) was not significantly different from the ratio of MVCNP/MVCP. When visual gain was altered, the mean ratio FNP/FP remained unchanged as compared to the veridical feedback condition (2.55), resulting in almost overlapping linear regression lines (Fig. 1).
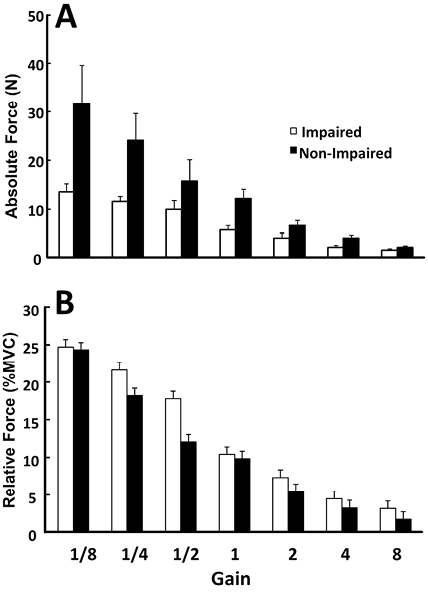
Even the largest changes in the visual gain (8 and 1/8) were not associated with significant changes in the force sharing between the two limbs, while the actual total force magnitude changed significantly (Fig. 2 and panel A of Fig. 3). When individual limb forces were normalized to corresponding MVCs, the relative forces of both limbs changed significantly and proportionally with visual gain alterations (Fig. 3B). A two-way ANOVA on normalized forces (panel B in Fig. 3) showed a significant effect of GAIN (F[6, 36] = 70.2, p < 0.001), but no main effect of SIDE and no GAIN × SIDE interaction. For example, when the non-impaired limb was made “weaker” with a small visual gain (1/8), subjects did not produce 80% MVC on the non-impaired side and 10%MVC on the impaired side to match the visual target of 10%MVCBI. Instead, they produced approximately 25%MVC by each limb (Fig. 3).
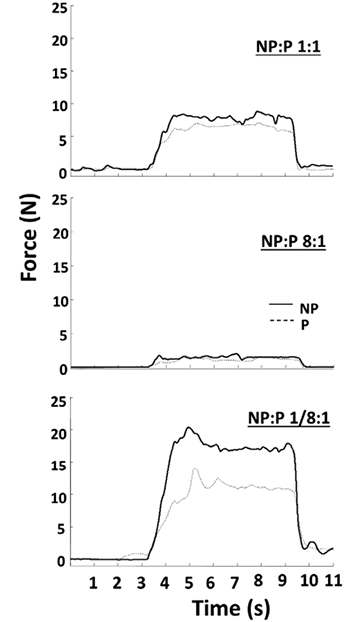
When visual gains to FNP were altered, the subjects were able to match the target accurately with the force feedback signal, FFB. While the subjects tended to overshoot the target in the trials with large gain values and to undershoot it when the gain value was low (Fig. 4A), no statistically significant effect of GAIN on matching error was found in a one-way ANOVA. There was a significant trend for the matching errors to decrease with the total force level. This logarithmic trend was clear for the veridical force feedback (gain = 1; R2 = 0.96, p < 0.01), as well as for the trials with altered gain values (R2 = 0.67; p < 0.05). The non-linear relation between the matching error and the actual total force was evidently shifted downward for altered visual gains (Fig. 4B). The tendency of undershooting with gain changes was the same for both high and low visual gains. When the errors were compared over the overlapping ranges of total force (3%–30%MVC) between the veridical gain condition and all conditions with changed gains, the latter value was significantly lower (t-test, p < 0.01)
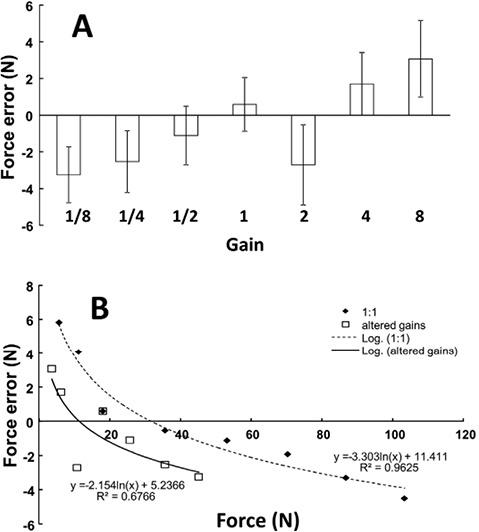
Fig. 4. Absolute matching errors remained statistically unchanged in the presence of altered visual gains (A). However, non-linear regression showed undershooting with altered visual feedback (B). Undershooting was statistically significant in the overlapping range of force.
Discussion
In the introduction, we hypothesized that altering visual gain for the force produced by one limb would change force output of that limb, and also the force produced by the contralateral limb via inter-limb interactions. This hypothesis has been supported by the data showing significant changes in the forces produced by both arms under changed visual gain conditions (Fig. 3A). The second hypothesis was that changes in the visual gain for one of the limbs would produce proportional changes in the force adjustments in both limbs. This hypothesis has also been confirmed (Fig. 3B).
Sharing pattern as a default
When a person is asked to produce a common mechanical effect with a redundant set of effectors, frequently a reproducible pattern of involvement of the effectors is observed. In particular, such stable sharing patterns have been described across a range of forces produced by fingers during multi-finger pressing tasks (15). A study with manipulations of visual gain for the force of one of the fingers showed that the sharing patterns remained largely unchanged (16). This allows suggesting that a sharing pattern may represent a default strategy possibly reflecting an optimization of an unknown cost function (17).
Deviations from a sharing pattern may be seen across repetitive trials at the same task; these deviations are commonly organized to stabilize (reduce variance of) a potentially important performance variable (reviewed in (18)). Such synergic adjustments have been reported in force production tasks by a redundant set of effectors including bilateral tasks (19, 20). A recent study has shown that these adjustments are organized in a subtle way reflected in different patterns of force co-variation when the visual feedback was computed using varying gains for the force of one of the effectors (21).
On the other hand, a few studies have shown that sharing patterns may change in the presence of altered visual feedback, even though these changes may be relatively subtle (5, 6). In our study, however, such deviations were not seen: The sharing pattern showed remarkable consistency over the whole range of total force values (for gain = 1) and gain values. We may conclude, therefore, that the rule “sharing is default” may be followed even more consistently in stroke survivors.
Strong interlimb coupling in stroke survivors
With normal visual feedback, our results showed that force contributions by the impaired and non-impaired limb to the total force remained constant across the tested range (3%–60% MVC). This finding is consistent with previous studies (7, 8). In the presence of altered visual gains to the non-impaired limb force, forces on both impaired and non-impaired limb changed proportionally (Fig. 1), such that relative contribution of each limb force to the total force remained unchanged (Fig. 3). Our results supported and extended previous findings (7–9) that there is strong interlimb coupling in hemiparetic stroke, which cannot be broken by distorted visual feedback.
Our results on the effects of visual gain manipulations in stroke survivors are different from findings in healthy subjects (5, 6). In those studies, during which young, healthy subjects matched total force targets with bilateral isometric index finger adduction, the weighting coefficients of the two forces ranged from 0.05:1 to 1:0.05. The authors reported a nonlinear correlation between the force output ratios and the weighting coefficient ratios, in contrast to our results of constant force output ratios in the presence of different coefficient ratios. A few factors could potentially account for the different observations. First, the range of weighting coefficients (gains) was smaller in the present study that could conceal the difference in the force outputs between the two limbs. This seems unlikely given the very strong linear relationship between the two limb forces in the presence of altered visual gains; note that this relationship was nearly identical to that observed in conditions with the veridical gain (Fig. 1).
Biomechanical constraints could strengthen the interlimb coupling in bilateral elbow flexion tasks more than in bilateral index finger force production. As shown in our previous studies on finger force interactions in bimanual multi-finger tasks, the secondary moment of force acting on the trunk in the frontal plane is commonly minimized by increased force production from non-instructed fingers on the “weaker” side (involving fewer fingers, or weaker fingers) (9, 22–24). In the present study, bilateral upper extremities of stroke survivors were positioned in symmetrical positions with shoulders flexed and abducted to approximately 45°, elbows flexed to approximately 90°, and forearms/wrists in a neutral position. In such position, unilateral elbow flexion generates a strong moment of force about the vertical axis of the trunk. Given the pronounced strength asymmetry between the two limbs, significant resultant moments of force were acting on the trunk under normal visual feedback. In the presence of altered visual gains, the default sharing pattern was preserved thus making no attempt to alter (minimize) the resultant moment of force. This could be done, for example, in conditions when the gain at the force of the non-paretic limb was low thus allowing the use of a more equal force sharing between the two limbs. Our observation can be interpreted as an inability of stroke survivors to take advantage of modified visual feedback conditions. This could be a reflection of a factor that kept the default sharing pattern even at the expense of producing a relatively large secondary moment.
Implications of functional units in stroke rehabilitation
Our observations of strong interlimb force coupling suggest that both impaired and non-impaired arms form a single functional unit in bilateral tasks with sharing pattern as a default. Such a functional unit offers a simple rule that defines the contributions of the two arms to a range of tasks; it may also contribute to recovery of the more impaired arm, which is forced to produce a range of force. This concept of functional unit could be integrated into bilateral training for stroke rehabilitation. Bilateral training is usually performed at a relatively low level of activity (25, 26). It is known that the impaired side tends to be “lazy” (i.e., it produces less force in proportion to its MVC – force deficit) during bilateral tasks at low force levels (27). However, there is growing evidence that patients after stroke receive more neuromechanical and functional gains after high-intensity training (28). To facilitate force production on the impaired side at a high level of activation for subsequent functional gain, our paradigm could be adopted to encourage high force production on the impaired side in bilateral tasks. As demonstrated in the present study, the activation level on the impaired side could be forced to increase following the default sharing pattern, if visual gain for the force on the non-impaired side is reduced during target matching tasks. Similarly, change of gain on the non-impaired side could be realized via change of resistance during bilateral robotic training. On the other hand, this adaptive design lacks flexibility by favoring positive correlations between the two arm actions across tasks that may benefit from or be harmed by such a correlation. For example, two-hand object manipulation may benefit from negative correlation of the two forces (29). Lack of flexibility in such functional units may hurt synergic control of bilateral actions (30). Note that synergic control seems to be relatively spared after stroke during multi-joint single-arm actions (31). Whether there is a qualitative difference in the response to stroke between unilateral and bilateral actions remains to be explored. We have to admit limitations of the study, in particular the relatively small sample size and the relatively large range of time after stroke in the subjects. On the other hands, significant effects observed in such a small group suggest that the effect size is large and may be of significant clinical value.
Conclusion
In summary, our findings indicate that there is a strong interlimb force coupling in hemiparetic stroke even in the presence of distorted visual feedback. Such strong interlimb force coupling suggests that both impaired and non-impaired arms form a single functional unit in bilateral tasks with sharing pattern as a default. The strong interlimb coupling may be utilized to facilitate recovery of the impaired limb in bilateral tasks at high intensities.
Acknowledgement
This study was supported in part by NIH grants (NIH/NINDS R01NS060774; NIH/NICHD/NCMRR R24 HD050821-08 under subcontract with Rehabilitation Institute of Chicago). The authors thank Jasper Yen, PhD for his assistance in data collection of some subjects and data analysis.
References
