Jeong Pyo Seo, MS and Sung Ho Jang, MD
From the Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daemyungdong, Namku, Taegu, Republic of Korea
OBJECTIVE: To report on a patient found to have injury of the spinothalamic tract on diffusion tensor tractography following traumatic brain injury.
Case description: A 29-year-old male patient with head trauma resulting from a pedestrian car accident presented with pain in multiple areas (both subscapular areas, posterior head and neck, both upper trapezius areas, and the right arm and leg). His pain had not improved with various types of conservative management.
RESULTS: Evaluations (conventional brain magnetic resonance imaging, electromyography, and whole spine magnetic resonance imaging), performed 2 years after the head trauma, did not reveal any specific abnormality. Fibromyalgia and myofascial pain syndrome were ruled out by physical examination. Injuries of the spinothalamic tracts in both hemispheres were observed on diffusion tensor imaging in terms of the configuration (thinning) and diffusion tensor tractography parameters (decreased fractional anisotropy or tract volume).
CONCLUSION: Some of the pain in the trunk and extremities in this patient could be ascribed to central pain caused by injury of both spinothalamic tracts. We conclude that diffusion tensor tractography provides a useful means of detecting injury of the spinothalamic tract in patients with traumatic brain injury.
Key words: spinothalamic tract; diffusion tensor imaging; head trauma, traumatic axonal injury.
J Rehabil Med 2014: 46: 00–00
Correspondence address: Sung Ho Jang, Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University 317-1, Daemyungdong, Namku, Taegu, 705-717, Republic of Korea. E-mail: strokerehab@hanmail.net.
Accepted Nov 18, 2013; Epub ahead of print Feb 27, 2014
Introduction
Prevalence of pain greater than 50% has been reported in patients with traumatic brain injury (TBI) (1, 2). Therefore, exact diagnosis and management of pain in patients with TBI is clinically important, and, for this, elucidation of the aetiology of pain is necessary. Pain in TBI can be caused by various pathoetiologies, including musculoskeletal, vascular, neurogenic, visceral, and iatrogenic mechanisms (3). Central pain is a form of neuropathic pain caused by a lesion in the brain or spinal cord (4). The pathogenetic mechanism of central pain has been relatively well elucidated in stroke; however, little is known about pain in TBI (4–6).
In the human brain, there are two main somatosensory pathways: the medial lemniscus and the spinothalamic tract (STT). The STT carries information on pain and touch from the contralateral extremities and body (7). Injury of the STT has been suggested as the pathogenetic mechanism of central pain. In the past, identification and visualization of the STP in the live human brain has been impossible. The recent development of diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), allows 3-dimensional visualization and estimation of the STT (8, 7). Many studies using DTT have reported that injury of the STT is related to the pathogenesis of central pain in stroke patients; however, no study in patients with TBI has been reported (9–11).
We report here on a patient with injury of the STT, shown on DTT, following head trauma.
Case Report
One patient and 11 age- and sex-matched normal control subjects (11 males; mean age 28.09 years, range 26–30 years) with no history of neurological disease were recruited for this study. All subjects provided signed, informed consent, and our Institutional Review Board approved the study protocol.
A 29-year-old right-handed male with no previous history of neurological, physical, or psychiatric illness had head trauma (acceleration and deceleration injury) resulting from a pedestrian car accident. The patient experienced a dazed feeling for approximately 5 s at the time of head trauma and this feeling continued for several minutes to a lesser degree; however, he did not experience loss of consciousness or post-traumatic amnesia. He was admitted to a local hospital immediately after the car accident, with a Glasgow Coma Scale score of 15. He began to experience pain in his right shoulder approximately 4–5 days after the trauma, and felt severe pain in multiple areas, including the right chest, the posterior head and neck, both areas of the upper back, and the right arm and leg since about 2–3 weeks after trauma, which he had not experienced before the car accident. He was diagnosed with a herniated cervical disc and internal lumbar disc disruption at the orthopaedic surgery department of a university hospital. He had received a cervical interlaminar epidural steroid injection several times and was prescribed opioid analgesics (hydromorphone 8 mg for approximately 1 year and, subsequently, oxycodone 10 mg/naloxone 5 mg for approximately 3 months before admission to our hospital, respectively). However, his pain had not shown improvement. In addition, he also reported memory dysfunction and concentration difficulty. He was referred to the psychiatry department at the same university hospital, and was diagnosed with anxiety disorder at approximately 6 months after onset. Two years after onset, he was admitted to the rehabilitation department of another university hospital. He reported pain on the posterior head and neck, both upper trapezius and subscapular areas, and the right arm and leg. The characteristics and severity of pain were as follows: throbbing pain in both subscapular areas, which was characterized by allodynia and hyperalgesia (visual analogue scale (VAS) score 6–7) (12), constant tingling and throbbing sensation in the posterior neck region with a lancinating sensation in both upper trapezius areas (VAS score 6), numbness and throbbing sensation on the posterior head (VAS score 5), tingling sensation on the right lateral leg (VAS score 5) with a sharp lancinating pain in the right sole (VAS score 7), and a constant burning sensation in the right hand and migrating shooting pain in the medial forearm (VAS score 6). Somatosensory function was determined using the subscales for tactile sensation and kinesthetic sensation of the Nottingham Sensory Assessment, Semmes-Weinstein monofilament, and moving 2-point discrimination were normal (13, 14). Myofascial pain syndrome or fibromyalgia were ruled out by physical examination. Brain magnetic resonance images (MRI) did not reveal any specific focal lesion, and an electromyography study showed no evidence of any peripheral nerve injury or radiculopathy. No abnormality was observed on cervical, thoracic, and lumbar spine MRI.
Diffusion tensor imaging
DTI data were acquired 2 years after onset using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips Ltd, Best, The Netherlands) by single-shot echo-planar imaging. For each of the 32 non-collinear diffusion sensitizing gradients, 70 contiguous slices parallel to the anterior commissure-posterior commissure line were acquired. Scanning was performed from the cortex to the middle of the second cervical vertebral body. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192 matrix; field of view = 240 × 240 mm; TR = 10,398 ms; TE = 72 ms; parallel imaging reduction factor (SENSE factor) = 2; EPI factor = 59; b = 1000 s/mm2; NEX = 1; slice gap = 0 mm and a slice thickness of 2.5 mm (acquired isotropic voxel size 2.5 × 2.5 × 2.5 mm). The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl) was used for analysis of diffusion-weighted imaging data, and affine multi-scale 2-dimensional registration was used to correct for head motion effects and image distortions due to eddy currents. Fibre tracking was performed using a probabilistic tractography method based on a multi-fibre model, and applied using tractography routines implemented in FMRIB Diffusion (5000 streamline samples, 0.5 mm step length, curvature threshold = 0.2). The STTs were identified by selection of fibres passing through both regions of interest (ROIs). A seed ROI was given in the posterolateral medulla on an axial slice and a target ROI was placed at the primary somatosensory cortex on an axial slice (8). We measured DTI parameters (fractional anisotropy (FA) and mean diffusivity (MD)) of the whole STT. DTI parameters showing a deviation of more than 2 standard deviations (SD) of that of normal control values were defined as abnormal. Out of 5,000 samples generated from each seed voxel, results for each contact were visualized threshold and weightings of tract probability at a minimum of one streamline through each voxel for analysis. Values of FA, MD, and tract volume of the STTs were measured. For measurement of inter-observer and intra-observer reliability, random analyses of the data were performed by 2 evaluators (SHJ and JPS) who were blinded to the other evaluator’s data. Intraclass correlation coefficient (ICC) was used to evaluate inter-observer and intra-observer reliability. We also reconstructed the DTTs for the corticospinal tract, fornix, cingulum, and optic radiation of the patient (Fig. 1).
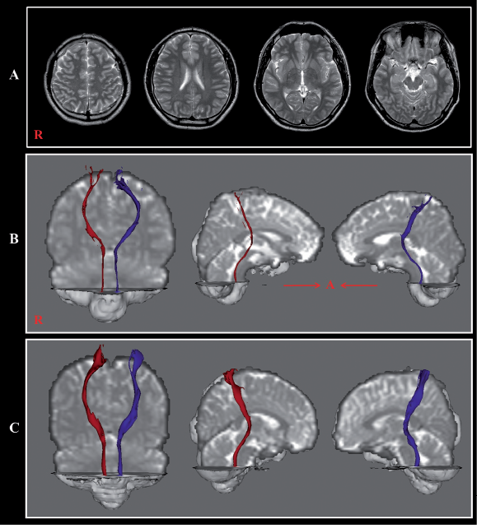
Fig. 1. (A) Brain magnetic resonance (MR) images obtained 2 years after onset showing no abnormality. (B) Reconstructed spinothalamic tract in both hemispheres (right: red colour; left: blue colour). (C) Reconstructed spinothalamic tract of a control subject (29-year-old male).
The STTs of both hemispheres in the patient were thinner than those of normal control subjects, although the integrity of the STTs was preserved from the cerebral cortex to the medulla in both hemispheres. A summary of FA and MD values of the STTs of the patient and normal control subjects is shown in Table I. The FA values of the right and left STT were more than 2 SD higher and lower than those of normal control subjects, respectively. The tract volume of the right STP was more than 2 SD lower than that of normal control subjects. However, the MD values of both STTs were similar to those of normal control subjects. On the other hand, the patient showed axonal injuries of both cingulums and the left optic radiation. The result of reliability showed good intraobserver (ICC = 0.96–0.99) and interobserver (ICC = 0.93–0.95) reliability.
|
Table I. Diffusion tensor image parameter values of the spinothalamic tracts of the patient and control subjects |
|||
|
FA |
MD |
Tract volume |
|
|
Patient |
0.446a |
0.820 |
401a |
|
Right |
|||
|
Left |
0.359a |
0.887 |
985 |
|
Controls, mean (SD) |
0.406 (0.017) |
0.889 (0.054) |
1,625.8 (606.0) |
|
aMore than 2 SD of that of normal control values. SD: standard deviation; FA: fractional anisotropy, MD: mean diffusivity. |
|||
Discussion
The current study analysed the STT of a patient who had experienced pain for 2 years after head trauma. The results showed: (i) both STTs of the patient were thinner in gross appearance than those of normal control subjects; (ii) the FA value was increased in the right STT and was decreased in the left STT; (iii) the MD values of both STTs were similar to those of normal control subjects; and (iv) the tract volume of the right STT was decreased, compared with those of normal control subjects. The FA value indicates the degree of directionality of water diffusion and has a range of zero (completely isotropic diffusion) to 1 (completely anisotropic diffusion) (15). It represents the white matter organization: in detail, the degree of directionality and integrity of white matter microstructures, such as axon, myelin, and microtubule, and the MD value indicates the magnitude of water diffusion (15). By contrast, the tract volume indicates the volume of the remaining STT. Therefore, these results (thinning of both STTs in gross appearance, decreased tract volume of the right STT, and decreased FA value of the left STT) appear to indicate injury of both STTs in this patient. On the other hand, this patient showed axonal injuries in both cingulums, which were reported as traumatic axonal injuries in other studies (16). We believe that traumatic axonal injury is the most plausible mechanism for injuries of both STTs. Some of the pain in the trunk and extremities in this patient could be ascribed to central pain caused by injury of both STTs. Although the patient reported pain with the characteristics of neuropathic pain, physical examination, electromyography, and brain and spine MRI did not reveal any other specific causes for this pain, other than injury of the STT.
There is scarce research on central pain in patients with TBI (4, 5). These studies have reported on the clinical characteristics or treatment of patients with central pain (4, 5). Since the introduction of DTI, several DTT studies have found that STP injury is related to the pathogenesis of chronic postsurgical pain (9–11). For TBI, a patient with TBI with a thalamic lesion in the ventroposterolateral nucleus, which was shown by the reconstructed DTT for the STT, was reported (6). The patient, who had had a motor vehicle accident, presented with a persistent tingling sensation and pain in her right upper and lower extremities; DTI showed that the FA values of the left STP at the thalamus were more decreased than the right STP.
In summary, we describe here a patient who was shown to have injury of both STTs on DTT following head trauma. Despite having undergone various evaluations and management, this patient with mild TBI had had severe pain for 2 years without accurate evaluation of the STT (17). Therefore, we recommend evaluation of the STT using DTT when a patient with head trauma reports pain with the characteristics of neuropathic pain or is refractory to routine management for pain, even in mild TBI. We believe that the described method would be helpful in detection of injury of the STT in patients with TBI. However, this study is limited because it was based on a single case, which was performed using low-tesla MRI machine. Further studies including larger numbers of patients, using a high-tesla MRI machine, are warranted. On the other hand, limitations of DTI should be considered (18, 19). Although DTI is a powerful anatomical imaging tool that can demonstrate the fibre architecture, it has significant limitation in reconstruction of neural tracts, due to partial volume effect and crossing fibre, which can prevent full reflection of the fibre architecture (18, 19).
Acknowledgements
This work was supported by the DGIST R&D Program of the Ministry of Education, Science and Technology of Korea (14-BD-0401).
References
