Jeong Pyo Seo and Sung Ho Jang
From the Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Republic of Korea
OBJECTIVE: We describe here a patient with intracerebral haemorrhage who showed recovery of an injured medial lemniscus and its related thalamocortical pathway on follow- up diffusion tensor tractography.
CASE REPORT: A 48-year-old man presented with right hemiplegia following a spontaneous intracerebral haemorrhage in the left corona radiata and basal ganglia. He underwent conservative management for intracerebral haemorrhage and comprehensive rehabilitative therapy.
RESULTS: The kinesthetic sensation score (maximum score 24 points) of the Nottingham Sensory Assessment improved from 6 points (at 2 weeks after injury) to 10 points (at 6 weeks) and to 18 points (at 12 weeks). For the left thalamocortical pathway, a discontinuation at the left midbrain below the haematoma was observed on the 2-week diffusion tensor tractography. The 6-week diffusion tensor tractography showed that the integrity of the left thalamocortical pathway had been restored to the left primary motor cortex, and the 12-week diffusion tensor tractography showed restoration to the left primary somatosensory cortex. The fibre number of the left thalamocortical pathway showed an increase (470 at 2 weeks after injury, 1,080 at 6 weeks, and 1,626 at 12 weeks).
CONCLUSION: This patient underwent recovery of an injured thalamocortical pathway over a period of 10 weeks after the second week following intracerebral haemorrhage, in terms of restoration of discontinued integrity and increased fibre number in the thalamocortical pathway.
Key words: diffusion tensor imaging; medial lemniscus; intracerebral haemorrhage; rehabilitation.
Correspondence address: Sung Ho Jang, Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, 705-717 Daegu, Republic of Korea. E-mail: strokerehab@hanmail.net
J Rehabil Med 2014; 46: 475–478
Accepted Jan 8, 2014; Epub ahead of print Apr 8, 2014
Introduction
Somatosensory dysfunction is common after stroke and can lead to functional impairment; somatosensory function therefore has important implications for stroke patients (1, 2). Elucidation of recovery mechanisms in stroke patients would provide important guidance for rehabilitation. Many studies have investigated the mechanisms of recovery of various functions in stroke patients, focussing in particular on motor function (3). However, relatively little is known about the mechanism of recovery of somatosensory function (2, 4–9).
There are two main somatosensory tracts in the human brain: the medial lemniscus and its related thalamocortical pathway (ML), and the spinothalamic tract and its related thalamocortical pathway (STT). The ML is responsible for proprioception, which results in the conscious awareness of body position in space, and the STT is the neural tract responsible for pain and body temperature. Many recent studies using diffusion tensor tractography (DTT) have reported on the clinical usefulness of DTT for the ML and STT, as well as methods used for identification of these tracts (10–12). However, little is known about the mechanisms of recovery of these somatosensory tracts (8).
We report here on a patient who showed recovery of an injured ML during a period of 3 months after an intracerebral haemorrhage (ICH), as demonstrated by follow-up DTT.
Case report
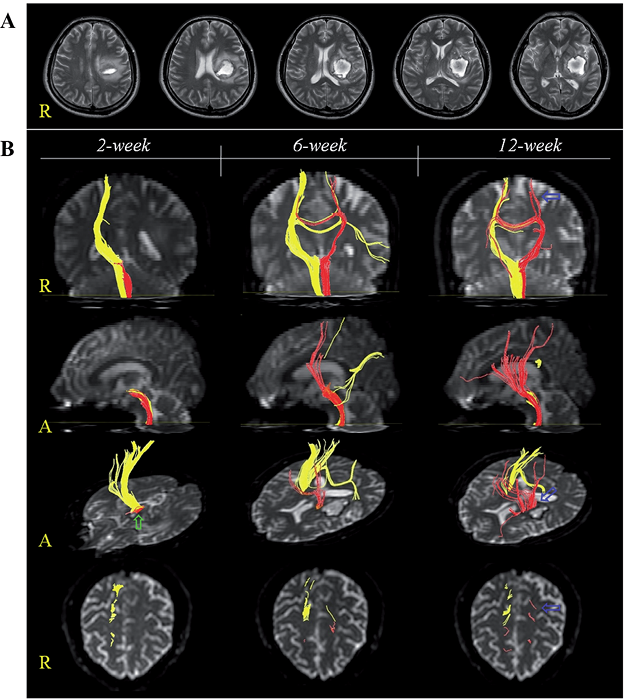
A 48-year-old, right-handed man presented with right hemiplegia, which occurred at the onset of a spontaneous ICH. He underwent conservative management for ICH at the department of neurosurgery of a university hospital. Two weeks after onset of ICH, he was transferred to the rehabilitation department of the same university hospital in order to undergo rehabilitation. Brain magnetic resonance images 2 weeks after onset showed a haematoma in the left corona radiata and basal ganglia (Fig. 1A). The patient received comprehensive rehabilitative therapy, including neurotrophic drugs (ropinirole, levodopa, and amantadine), movement therapy, somatosensory stimulation, and procedures for spasticity control of the left finger flexors (13, 14). Movement therapy and somatosensory stimulation focused on improvement of sensori-motor function of the right upper and lower extremities and was performed during the physical and occupational therapy sessions, 5 times per week (140 min/day). The subscale for kinesthetic sensation (maximum score 24 points) of the Nottingham Sensory Assessment (NSA) was used for determination of somatosensory function (15). The kinesthetic sensation score of the NSA improved from 6 points (at 2 weeks after onset) to 10 points (at 6 weeks), then to 18 points (at 12 weeks). Weakness of the patient’s right extremities improved from a Motricity Index of 9 points at 2 weeks to 49 points (at 6 weeks) and 56 points (at 12 weeks) (16). As a result, the patient was able to walk independently on an even floor at 6 weeks and ascend and descend stairs at 12 weeks after onset; however, due to severe injury of the left corticospinal tract, he could not perform fine motor activities with his right hand.
The patient provided signed, informed consent and the study protocol was approved by our institutional review board.
Diffusion tensor TRACTOGRAPHY imaging
DTT images were acquired 3 times (at 2, 6, and 12 weeks after onset) using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips Ltd, Best, The Netherlands) with single-shot echo-planar imaging. For each of the 32 non-collinear diffusion sensitizing gradients, 70 contiguous slices were acquired parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192 matrix, field of view = 240 × 240 mm, repetition = 10,398 ms, echo time = 72 ms, parallel imaging reduction factor (SENSE factor) = 2, echo-planar imaging factor = 59 and b = 1000 s/mm, number of excitations = 1, and a slice thickness of 2.5 mm (acquired isotropic voxel size 2.5 × 2.5 × 2.5 mm). Removal of eddy current-induced image distortions using affine multi-scale 2-dimensional registration was performed at the Oxford Centre for Functional Magnetic Resonance Imaging of Brain Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Reconstruction of the MLs was performed using DTI-Studio software (CMRM, Johns Hopkins Medical Institute, Baltimore, MD, USA). MLs were determined by selection of fibres passing through 2 regions of interest (ROIs). The first ROI was located in accordance with known anatomy at the level of the medulla (the portion medio-posterior to the pyramid). The second ROI was at the posterior part of the upper pons (blue colour) on the colour map (12). Fibre tracking was started at any seed voxel with a fractional anisotropy (FA) > 0.2 and ended at a voxel with a FA of < 0.2 and a tract turning-angle of < 60º. The values of FA, apparent diffusion coefficients (ADC) and fibre number (FN) were measured.
The right ML originated from the right cerebral cortex, including the primary somatosensory cortex (S1), which passed along the known ML pathway (Fig. 1B). The 2-week DTT for the left ML showed a discontinuation at the left midbrain below the haematoma. The 6-week DTT showed that the integrity of the left ML had been restored to the left primary motor cortex; however, it was not connected to the left S1. On the 12-week DTT, the trajectories of the left ML were increased and the integrity to the left S1 was restored (Fig. 1B). Although the FA and ADC values of the DTTs for the left ML were similar, the FNs were increased (470 at 2 weeks, 1,080 at 6 weeks, and 1,626 at 12 weeks) (Table I).
|
Table I. Changes in clinical data and diffusion tensor tractography (DTT) imaging parameters |
|||
|
2 weeks |
6 weeks |
12 weeks |
|
|
Clinical data |
|||
|
NSA (1–24) |
6 |
10 |
18 |
|
MI (1–100) |
9 |
49 |
56 |
|
DTT imaging parameters |
|||
|
FA |
0.49 |
0.49 |
0.46 |
|
ADC |
0.85 |
0.79 |
0.80 |
|
FN |
470 |
1,080 |
1,626 |
|
NSA: subscale for kinesthetic sensation of the Nottingham Sensory Assessment; MI: Motricity Index; FA: fractional anisotropy; ADC: apparent diffusion coefficient; FN: fibre number. |
|||
Discussion
This patient underwent a process of recovery of an injured ML during a period of 10 weeks after the second week following ICH, in terms of restoration of discontinued integrity and increased FN of the injured ML, and clinical recovery of proprioception on the affected side. Regarding the integrity of the injured ML, the left ML appeared to be severely injured, as shown by 2-week DTT, in terms of a discontinuation at the midbrain level below the haematoma. On follow-up DTTs, the integrity of the discontinued left ML was shown to be restored to the left primary motor cortex at 6 weeks after symptom onset, and to the left S1, which is the main origin of the ML, at 12 weeks after onset. The FN of the left ML showed an increase over time at 2 weeks (470), 6 weeks (1,080), and 12 weeks (1,626), with no significant changes in the FA and ADC values. The FA value represents the degree of directionality of microstructures such as axon and myelin, and the ADC value indicates the magnitude of water diffusion (17). The FN reflects the total number of fibres in a neural tract (17). Therefore, the increment of FN of the left ML suggests the increment of FN of the injured left ML. In addition, clinical recovery of proprioception on the NSA subscale for the right side (a score of 6 at 2 weeks, 10 at 6 weeks, and 18 at 12 weeks) appears to provide additional evidence of the recovery of the injured left ML.
The mechanisms of recovery of somatosensory function in stroke patients are classified as follows: recovery of an injured somatosensory pathway, peri-lesional reorganization, contribution of the unaffected somatosensory cortex, contribution of the secondary somatosensory cortex, and recovery mechanisms in patients with thalamic lesion (2, 4–9). There have been few studies of the recovery of an injured somatosensory pathway in patients with stroke (8). In 2010, Hong & Jang (8) reported the recovery of somatosensory function in a patient with a spontaneous ICH in the right cortex and corona radiata. The patient presented with severe somatosensory dysfunction on the left side at onset, and the kinesthetic sensation score of the NSA (maximum score 24 points) improved from 12 points at 3 weeks to 18 points at 7 weeks. On the 3- and 7-week functional MRI, the ipsilesional cortex centred on the S1 was activated during proprioceptive input of the affected hand. Activation of ipsilesional S1 showed an increase on the 7-week fMRI, compared with that of the 3-week fMRI. The injured ML was not reconstructed on the 3-week DTT; however, the injured ML was reconstructed through the medial side of the lesion to the S1. By contrast, the patient described in this study showed more severe deficit of proprioception at initial evaluation and showed a different course of recovery (longer and poorer). In addition, the patient described in the previous study showed restoration to the ipsilesional S1 at 7 weeks after onset; in contrast, this patient showed restoration to the ipsilesional primary motor cortex at 6 weeks and then to the ipsilesional S1 at 12 weeks after onset (18).
In conclusion, we report here a patient who showed recovery of a severely injured ML after ICH in terms of clinical recovery and recovery of the integrity and FN of DTT. Because this case report demonstrated the process of recovery of a severely injured ML during the early phase following ICH, this provides information about recovery of somatosensory dysfunction, which is useful for stroke rehabilitation. Detailed knowledge about recovery mechanisms in stroke would provide the basis for establishment of scientific rehabilitation strategies. However, this study is limited as it is a single case report. Further complementary studies with larger numbers of patients are warranted. In addition, research is required into the effects of the rehabilitative modalities employed with this patient, including the neurotrophic drugs (which are known to be effective in motor recovery of stroke patients (13, 14)) on the recovery of an injured ML. The clinical significance of this kind of recovery of the ML also requires clarification. However, the limitations of DTT imaging, which may underestimate or overestimate of fibres of a neural tract should be taken into account (19).
AcknowledgeMENTs
This work was supported by the DGIST R&D Program of the Ministry of Education, Science and Technology of Korea (14-BD-0401).
References
