Marina Demetrios, MBBS1, Alexandra Gorelik, MSc2, Julie Louie, MPhys3, Caroline Brand, MBBS, MPH2,4, Ian J. Baguley, MBBS, PhD5 and Fary Khan, MBBS, MD1,6
From the 1Department of Rehabilitation Medicine, Royal Melbourne Hospital, 2Melbourne EpiCentre, The University of Melbourne, 3Department of Physiotherapy, Royal Melbourne Hospital, 4Centre for Research Excellence in Patient Safety (CREPS), Monash University, Melbourne, 5Brain Injury Rehabilitation Service, Westmead Hospital, Sydney and 6Department of Medicine, Dentistry and Health Sciences, The University of Melbourne, Melbourne, Australia
OBJECTIVE: To examine the benefits of high intensity ambulatory rehabilitation programmes over usual care following botulinum toxin A (BoNT-A) for post-stroke spasticity in Australian adults.
DESIGN: Prospective single centre, controlled clinical trial.
PARTICIPANTS: Fifty-nine adults, median 61 years old and 2.5 years following stroke.
METHODS: Participants were dichotomised into high intensity ambulatory rehabilitation programmes (≥ 3 × 1-h weekly sessions for approximately 10 weeks) or usual care programmes (≤ 2 × 1-h weekly sessions) following BoNT-A injections for spasticity. A blinded assessor completed outcomes at 0 (baseline), 6, 12 and 24 weeks. Primary endpoints: proportion of participants achieving ≥ 50% of their goals (using Goal Attainment Scaling: GAS) and GAS T-score change at 12 weeks. Secondary outcomes: Modified Ashworth Scale (MAS), participant satisfaction, activity/participation measures and caregiver burden.
RESULTS: Both groups showed significant improvement in goal attainment and participant satisfaction up to 24 weeks, with no overall between-group significant differences. There was, however, a statistical trend (p = 0.052) for participants to achieve more upper limb goals in the high intensity therapy group. GAS and satisfaction benefits persisted beyond the duration of spasticity reduction as measured by MAS.
Conclusions: While patient-centred outcomes following BoNT-A injections for post-stroke spasticity were not influenced by intensity of ambulatory rehabilitation programmes, there was a trend for high intensity therapy to be associated with greater upper limb goal attainment. This suggests that the effects of more intensive therapy may be a modifier of the ‘black box’ of rehabilitation; however, further research is required to evaluate this effect and determine which elements of therapy programmes optimise post-BoNT-A outcomes.
Key words: botulinum toxin; muscle spasticity; stroke; rehabilitation.
J Rehabil Med 2014; 46: 730–737
Accepted Mar 28, 2014; Epub ahead of print Jul 30, 2014
Correspondence address: Dr Marina Demetrios, Rehabilitation Medicine Physician, Royal Melbourne Hospital, Royal Park Campus, 34–54 Poplar Road, Parkville 3052, VIC, Australia. E-mail: Marina.Demetrios@mh.org.au
Introduction
Stroke is a leading cause of disability worldwide, with spasticity affecting up to 43% (1) of stroke survivors. The burden of post-stroke spasticity is high in terms of treatment costs, quality of life (QoL) consequences, caregiver burden and other health-related outcomes (2).
Post-stroke spasticity contributes to a diversity of patient-centred problems (3). These may relate to: ’impairments’ (problems with body structures or physiological function) such as restricted joint range of movement, pain and involuntary movements; ‘activity limitations’ (‘active’ and ‘passive’ function); and ‘restrictions in participation’ which limit societal involvement such as engagement in work, family roles and leisure activities. Spasticity can impact on ‘active’ function (the execution of a functional task by the individual) by restricting mobility and upper limb use during daily activities, and ‘passive’ function (caring for an affected limb), such as maintaining palmar hygiene or applying a splint. As spasticity affects individual stroke survivors differently, so treatment goals are variable. Hence, the use of functional outcome measures, such as Goal Attainment Scaling (GAS), that identify outcomes of importance to the individual and caregivers are recommended (3–6).
The effectiveness of botulinum toxin A (BoNT-A) in reducing spasticity following stroke has been well established in both upper (6–9) and lower limbs (10, 11). However, spasticity management utilises a multimodal rehabilitation approach to achieve long-term functional improvement after spasticity reduction (12). In this capacity, BoNT-A is considered an adjunctive intervention, with temporary effects (13), that provides a window of opportunity to maximise gains during a rehabilitation program. An appropriate rehabilitation management program should ideally be in place prior to BoNT-A treatment, and should continue thereafter (14). Whilst spasticity management guidelines advocate a multidisciplinary approach (15, 16), recommendations are based on expert opinion rather than scientific evidence. The guidelines lack details on optimal therapy programmes and patient selection.
Physical therapies used in rehabilitation following BoNT-A injections may include electrical stimulation (17), stretching (18), casting (19), constraint induced movement therapy (CIMT) (20, 21) and task-specific practice (22). A recent review found limited and low quality evidence for the effectiveness of multidisciplinary rehabilitation intervention following BoNT-A for post-stroke spasticity (23). The few included studies had methodological limitations including small sample sizes and use of outcome measures that do not necessarily translate into improved function or benefits for patients and caregivers. Studies of physical interventions following BoNT-A have tended to focus on single treatment modalities or uni-disciplinary therapy, rather than the complex array of interventions delivered in real-life rehabilitation settings. The optimal types (modalities, therapy approaches, settings) and intensities of therapies for achieving meaningful patient outcomes following BoNT-A remain unclear, causing these elements to be described as the ‘black box’ of rehabilitation (24).
Traditionally, stroke patients are told their recovery stabilizes within 6–12 months (25). These plateaus may be due to patient physiological or psychological adaptation to their rehabilitation exercises, rather than reduced capacity for motor recovery (26). Adjusting the rehabilitation approach (modifying intensity or modalities) and challenge of therapeutic exercise allows positive neuromuscular adaptations to occur (26). Additionally, there is often a time lag between peak spasticity reduction following BoNT-A and maximal functional gain (12). These factors emphasize the need for comprehensive rehabilitation and longer follow-up periods to ensure that the benefits of treatment are not missed and to determine whether effects are maintained after treatment cessation.
This study examined the effectiveness of a high intensity ambulatory multidisciplinary rehabilitation program versus lower intensity usual care, as measured by goal achievement and other outcomes up to 24 weeks, in Australian adult stroke survivors receiving BoNT-A injections for upper and/or lower limb spasticity.
Methods
Participants and setting
The study was conducted at a multidisciplinary, tertiary referral spasticity management service. Following ethics committee approval, consecutive adult patients treated with BoNT-A for upper and/or lower limb post-stroke spasticity and eligible for rehabilitation were invited to participate in the study. Inclusion criteria were: age ≥ 18 years, stroke diagnosis ≥ 3 months; upper and/or lower limb spasticity (MAS ≥ 2) interfering with function or causing a clinical problem and no contraindications to BoNT-A injections. Patients were excluded if they had: treatment with BoNT-A within 6 months, intrathecal baclofen or anti-spasticity medications; undergone neurolysis or surgery to the affected limb; concomitant neurological conditions; were pregnant; or were unable to participate in therapy due to cognitive or language impairment, psychiatric or medical illness.
Procedures
Group allocation. This trial was designed to reflect ‘real-life’ clinical practice in an ambulatory rehabilitation service in Australia. In this context, therapy is delivered based on accessibility to services, treating team assessments, and service delivery protocols determined by geographical catchment areas. Thus, allocation to rehabilitation programmes was based on participants’ areas of residence. Those residing within a 12-km radius of the investigating hospital received high intensity therapy, whilst subjects outside of this geographical catchment underwent usual care, being lower intensity therapy at their local community or hospital rehabilitation service. Participants were not informed of allocation within the trial.
Assessments. Structured assessments were completed in the hospital clinic. Baseline data included: demographic data collection, assessment (history, medical records, clinical examination) of stroke related impairments and prior BoNT-A administration. Up to 3 individualised, SMART (specific, measurable, achievable, realistic and timed) (27) functional goals for each treated limb (maximum 6 goals if both limbs were treated) were negotiated between participants, caregivers and therapist (JL). Using the GAS process (28), a defined ‘statement of expected outcome’ was determined for each goal at 12 weeks following BoNT-A injections. Participants were referred to ambulatory rehabilitation services based on geographical catchment areas and details of treatment goals were provided. A blinded assessor completed standardized outcome assessments (see outcome measures) at 0 (baseline), 6, 12 and 24 weeks.
Treatment schedules
BoNT-A injections. All participants received individualized BoNT-A injections in the affected limb/s, as determined by clinical factors, spasticity patterns, injector preference and treatment goals (15, 16). Injections were administered at baseline by 1 of 2 rehabilitation physicians using neuromuscular stimulation.
Rehabilitation programmes. A high intensity ambulatory rehabilitation program comprised of 3 or more 1-h sessions per week for approximately 10 weeks. This protocol was more intensive than the usual care provided for spasticity management in local tertiary hospital community based rehabilitation (CBR) services. Usual care was a lower intensity rehabilitation program (≤ 2 × 1-h sessions per week). Therapy settings included tertiary hospital CBR services or community health centres, depending on service accessibility and availability as per routine service delivery. A priori compliance with outpatient treatment was attendance in > 70% of scheduled therapy sessions.
All participants received goal-directed, individualized rehabilitation programmes, consistent with ‘real-life’ rehabilitation practices in Australia. Therapy was based on neurodevelopmental techniques. Interventions targeting relevant impairments were primarily: motor learning, strengthening, postural awareness, balance training, aerobic/ conditioning exercises, range of movement, stretching, adaptive/ compensatory strategies (environmental adaptation, one handed skills), task specific practice and sensory training. The main activities focussed on were gait or upper extremity control in relation to activities of daily living (dressing, feeding, cleaning, etc). Others included transfer practice, sitting balance, trunk control, and functional mobility. Participants received education in self-management and home exercise programmes. Therapists documented details of therapy sessions (discipline, date, duration, activities and interventions) using standardised forms (24).
Outcome measurement
Investigator-observed and participant (or caregiver) reported outcome measures were completed at 0 (baseline), 6, 12 (primary outcome time-point), and 24 weeks.
Primary outcome measures
The proportion of participants who at 12 weeks achieved at least 50% of their total goals, as measured by GAS process, and change in GAS T-scores (28), using methods described elsewhere (4, 5). Goals were identified using the goal-setting procedure and weighted by importance and difficulty (each graded on a 0–3 scale) of achieving the goal. Baseline goal scores were –1 (or –2 if participants could not have been at a worse level). Goal attainment was rated using a 5-point scale (–2 to +2), where a GAS score of 0 and above implies goal achievement. The blinded assessor assigned the level of individual goal attainment according to the description in the ‘statement of expected outcome’ (‘0’ score) at follow-ups. The composite goal attainment score (T-score), based on the aggregated weighted score of each participant’s goals, was calculated (28) as a standardized variable normally distributed around a mean of 50 and with a standard deviation (SD) of 10 points (5).
Secondary outcome measures
The Modified Ashworth Scale (MAS) (29) assessed muscle tone during passive range of movement of the joints associated with injected muscle groups in the treated limb/s (0 = no increase in muscle tone to 4 = rigid flexion or extension). A score of 1+ was assigned the value of 1.5. Change in mean MAS scores for treated limbs, and upper and lower limbs separately, were calculated.
The Arm activity measure (ArmA) (30) assessed difficulty in passive (7-items, section A) and active (13-items, section B) arm function for participants who had upper limb BoNT-A injections. Participants or their caregivers rated the difficulty in performing each function, based on activity over the preceding 7 days, using a 5-point ordinal scale (0 = no difficulty, 1 = mild, 2 = moderate, 3 = severe, 4 = unable to do).
The 10-m walk test (31) measured comfortable gait speed (m/s), with shoes and usual gait aid, for participants who had lower limb injections. The mean of two tests was used.
Global assessment scale (32) rated participants’ subjective improvement or worsening of symptoms and satisfaction following treatment (–4 = very marked worsening to 0 = no change to +4 = very marked improvement, ≥ +1 indicated treatment success).
Self-rated burden (SRB) (33) for caregivers used a visual analogue scale with numerical ratings in response to the question ‘How burdensome do you feel caring for your partner is at the moment?’; from 0 meaning it is ‘not hard at all’ to 100, meaning that it is ‘much too hard’.
Power calculation and statistical analysis
A change in GAS T-sore of at least 10 points is associated with clinically important change (4). Sample size was estimated by assuming a difference in GAS T-score change score of 10 (SD 12) points between high intensity and usual care groups. Allowing for a 15% dropout rate, 27 participants were required in each group to detect the difference with 80% power.
Data was entered into MS Access database and exported into Stata12 (StataCorp, TX, USA) for analysis. Descriptive statistics were presented as mean and SD for continuous normally distributed data, median and interquartile range (IQR) for skewed or ordinal data and n (%) for categorical data.
Data analysis was performed using intention-to-treat principles with Last Observation Carried Forward for missing data. Continuous normally distributed variables were analysed using Student t-test and skewed or ordinal data were analysed using Wilcoxon rank-sum test. Change scores were calculated as follow-up minus baseline scores. Categorical variables were analysed using Chi2 or Fisher’s exact tests. Multivariate logistic regression was used to determine variables associated with participants achieving at least 50% of their goals and generalized linear model was used to determine variables associated with the change in GAS T-score. Variables of interest included in models were: treatment allocation, age, gender, stroke localisation (cortical versus subcortical) and time since stroke (≤ 1 year versus > 1 year). p-values < 0.05 indicated statistical significance for all tests.
Results
Fifty-nine of 75 stroke survivors screened from January 2011 to June 2012 were recruited to the study, with 28 allocated to high intensity rehabilitation programmes and 31 to lower intensity usual care (Fig. 1). There were no losses to follow-up or adverse events.
Fig. 1. Study flow chart.
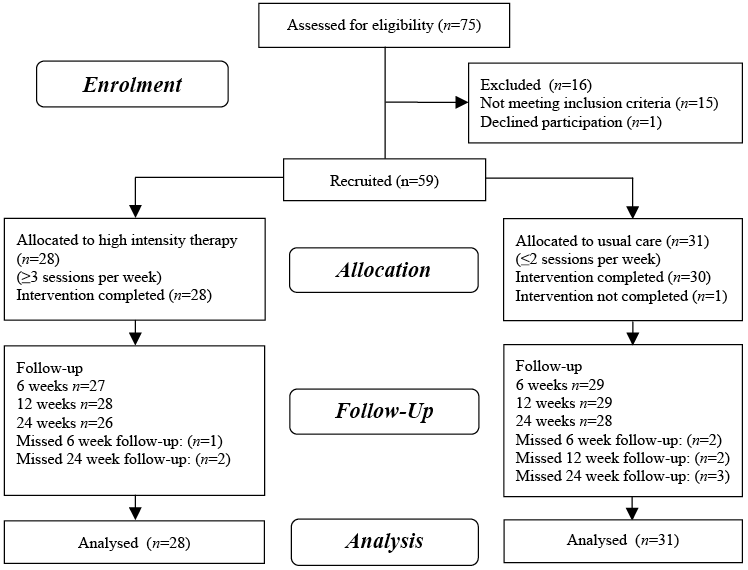
Baseline characteristics
Demographic and clinical factors for both groups are summarized in Table I. Participants had a median age of 61 years (IQR = 48–68) and a median time since stroke diagnosis of 2.5 years (IQR = 1.1–5.0). Over 70% (n = 42) were male. A greater proportion of usual care participants had cortical strokes (p = 0.036). However, there were no differences in the proportion of participants with cortical and cognitive impairments on assessment between the 2 groups. The high intensity group had lower ArmA active function score and GAS T-score (p = 0.027 and 0.053, respectively).
|
Table I. Baseline characteristics of high intensity and usual care groups |
||
|
High intensity (n = 28) |
Usual care (n = 31) |
|
|
Demographic factors |
|
|
|
Sex male, n (%) |
19 (67.9) |
23 (74.2) |
|
Age, years, mean (SD) Range |
60.6 (48.6–65.9) 20.3–83.1 |
61.4 (47.8–68.6) 37.4–78.5 |
|
Time since stroke, years, mean (SD) Range |
2.3 (1.1–5.5) 0.4–15.3 |
2.5 (1.1–5.0) 0.3–12.0 |
|
Education, n (%) Primary Secondary Tertiary/other |
1 (3.6) 18 (64.3) 9 (32.1) |
6 (19.4) 14 (45.2) 11 (35.5) |
|
Living arrangements, n (%) With family/friends Alone Other |
25 (89.3) 1 (3.6) 2 (7.1) |
27 (87.1) 3 (9.7) 1 (3.2) |
|
Caregiver(s), n (%) |
21 (75.0) |
26 (83.9) |
|
Clinical characteristics |
|
|
|
Stroke aetiology, n (%) Infarct Haemorrhage/mixed |
20 (71.4) 8 (28.6) |
25 (80.6) 6 (19.4) |
|
Stroke localisation, n (%) Cortical Subcortical |
20 (71.4) 8 (28.6) |
29 (93.5)* 2 (6.5) |
|
Left cerebral lesion, n (%) |
16 (57.1) |
17 (54.8) |
|
Dominant side affected, n (%) |
18 (64.3) |
15 (48.4) |
|
Dependent variables |
|
|
|
GAS T-score, median (IQR) |
31.3 (26.9–36.1) |
36.4 (31.0–37.2) |
|
MAS treated limb/s, median (IQR) All (UL & LL) (n = 59) UL (n = 40) LL (n = 37) |
2.4 (2.0–2.7) 2.4 (2.0–2.7) 2.3 (2.0–3.0) |
2.4 (2.0–2.7) 2.1 (2.0–3.0) 2.5 (2.0–3.0) |
|
ArmA, median (IQR) |
(n = 21) |
(n = 19) |
|
Section A Section B |
9 (6–13) 46 (42–48) |
10.5 (6–15) 49.5 (46–51)* |
|
Gait speeda, m/s, median (IQR) |
(n = 16) |
(n = 20)b |
|
0.37 (0.29–0.74) |
0.33 (0.18–0.58) |
|
|
Prior treatments |
|
|
|
Therapy program pre-existing and continued, n (%) |
18 (64.3) |
17 (54.8) |
|
BoNT-A > 6 months prior to recruitment, n (%) Cycles, n, median (IQR) |
16 (57.1) 1.0 (1–3) |
15 (48.4) 2.5 (1–4) |
|
*p < 0.05. aGait speed (m/s) for participants receiving LL injections. bOne participant unable to complete 10-m walk test. GAS: Goal Attainment Scaling; MAS: Modified Ashworth Scale (mean score for treated muscle groups in limb/s, scoring = 1, 1.5, 2, 3, 4); UL: upper limb; LL: lower limb; ArmA: arm activity measure (section A ‘passive’ and section B ‘active’ arm function) for participants receiving UL injections; OT: occupational therapy; PT: physiotherapy; BoNT-A: botulinum toxin A; IQR; interquartile range; SD: standard deviation. |
||
BoNT-A injections
Forty participants received upper limb (UL) injections (21 in high intensity and 19 in usual care groups) and 37 received lower limb (LL) injections (16 in high intensity and 21 in usual care groups). Nine participants in each group had both limbs injected. There were no significant differences in the proportions of participants who had UL and LL injections in each group (p = 0.40 and 0.26, respectively). Fifty-four participants were injected with Dysport® (Ipsen Ltd, Slough, UK) and 5 with Botox® (Allergan Inc., Irvine, USA). Mean doses of BoNT-A (Dysport®) were 766 (SD 244) in the high intensity and 673 (SD 314) in the usual care groups (p = 0.19). There were no significant differences between UL and LL doses between groups (p = 0.19 and 0.94, respectively). Elbow flexors and long finger flexors were most commonly injected in the UL, and gastrocnemius and/or soleus were injected in over 90% of those receiving LL injections (Table II).
|
Table II. Number of participants injected in the various upper and lower limb muscle groups |
||
|
High intensity (n = 28) n (%) |
Usual care (n = 31) n (%) |
|
|
Upper limb injections |
(n = 21) |
(n = 19) |
|
Shoulder muscles: pectoralis, latissimus dorsi, corachobrachialis |
4 (19.0) |
5 (26.3) |
|
Elbow flexors: biceps, brachialis, brachioradialis |
12 (57.1) |
11 (57.9) |
|
Pronators: pronator teres, pronator quadratus |
8 (38.1) |
2 (10.5) |
|
Wrist flexors: flexor carpi ulnaris, flexor carpi radialis |
14 (66.7) |
6 (31.6) |
|
Finger flexors: flexor digitorum superficialis, flexor digitorum profundus |
15 (71.4) |
13 (68.4) |
|
Thumb: flexor pollicus longus, adductor pollicus, flexor pollicus brevis, opponens pollicus |
11 (52.4) |
8 (42.1) |
|
Lower limb injections |
(n = 16) |
(n = 21) |
|
Quadriceps: vastus intermedius, rectus femoris |
3 (18.8) |
5 (23.8) |
|
Plantarflexors: gastrocnemius medial, gastrocnemius lateral, soleus |
15 (93.8) |
20 (95.2) |
|
Invertors: tibialis posterior, tibialis anterior |
6 (37.5) |
4 (19.0) |
|
Toe flexors: flexor hallicus longus, flexor hallicus brevis, flexor digitorum brevis, flexor digitorum longus |
2 (12.5) |
4 (19.0) |
|
n is the number of participants injected in ≥ 1 of the muscles in the muscle group and calculated as a % of those injected in the upper or lower limb for high intensity vs usual care groups. Total is > 100% as participants had injections in multiple locations. |
||
Rehabilitation programmes
Therapy commenced within 14 days of BoNT-A injections for the majority of participants. Participants in the high intensity group attended a mean of 3.2 × 1-h therapy sessions per week (SD 0.6, range 2–4), and the usual care group an average of 1.2 (SD 0.5, range 0.7–2.2). Mean duration of therapy programmes was 11 weeks (SD 3.3, range 7.9–19.4) for the high intensity group and 10 weeks (SD 3.9, range 1–20.3) for the usual care group. All participants attended hospital CBR services for therapy programmes, except for 3 in the usual care group who attended community health centres (n = 2) and a private therapist (n = 1). Rehabilitation programmes included physiotherapy (96.4% of high intensity and 87.1% of usual care participants), occupational therapy (64.3% and 48.4%, respectively) or at least 2 disciplines (64.3% and 48.4% respectively). A priori compliance was achieved in all but one participant (usual care group) who declined to participate in therapy after one session.
Goals
There were 93 goals set in the high intensity and 96 in the usual care groups (mean 3 goals per participant, range 1–6), with the majority of goals related to the ICF domains of activity/participation rather than symptoms/impairments (Table III).
|
Table III. Categories of upper and lower limb goals for high intensity and usual care groups |
||
|
Goal categories |
High intensity n (%) |
Usual care n (%) |
|
UL goalsa |
||
|
Impairments/symptoms |
||
|
Pain/discomfort |
4 (7.1) |
6 (13.6) |
|
ROM/prevent contracture |
6 (10.7) |
3 (6.8) |
|
Involuntary movements |
6 (10.7) |
7 (15.9) |
|
Activity/Participation |
||
|
Active function |
21 (37.5) |
12 (27.3) |
|
Passive function |
18 (32.1) |
15 (34.1) |
|
Mobility |
1 (1.8) |
1 (2.3) |
|
Total UL goals, n |
56 |
44 |
|
LL goalsa |
||
|
Impairments/symptoms |
||
|
Pain/ discomfort |
1 (2.7) |
6 (11.5) |
|
Involuntary movements |
0 (0.0) |
2 (3.8) |
|
Activity/Participation |
||
|
Active function |
34 (91.9) |
44 (84.6) |
|
Passive function |
1 (2.7) |
0 (0.0) |
|
Other |
1 (2.7) |
0 (0.0) |
|
Total LL goals, n |
37 |
52 |
|
a% of UL or LL goals out of the total number of goals set for high intensity and usual care groups. UL: upper limb; LL: lower limb; ROM: range of movement. |
||
Primary outcomes
The majority of participants in high intensity and usual care groups achieved at least 50% of their goals at 12 weeks (75.0% and 77.4%, respectively, p = 0.999) and 24 weeks (78.6% and 61.3%, respectively, p = 0.170). GAS T-scores improved significantly (p < 0.001) at all time points in both groups. When considering all goals, median change in GAS T-score from baseline to 24 weeks approached statistical significance favouring the high intensity vs usual care group (20.1 (IQR 10.4–25.5) vs 12.3 (IQR 5.8–18.7), respectively, p = 0.071) (Table IV). However, analysing goal attainment by upper and lower limb GAS revealed a strong statistical trend towards participants with UL injections achieving more goals at 24 weeks in the high intensity compared to the usual care group (median 3 (IQR 1–3) versus 1 (IQR 1–2), p = 0.052), with no observed difference for those who had LL injections.
Secondary outcomes
The high intensity group showed greater reduction in mean UL MAS score compared with the usual care group at 6 (p = 0.005) and 12 (p = 0.015) weeks, and overall mean MAS score at 12 weeks (p = 0.033) (Table IV). The usual care group had a greater reduction in LL MAS score at 24 weeks (p = 0.004). Overall, MAS scores trended towards baseline at 12 and 24 weeks. Participant satisfaction with treatment, measured using the global assessment scale, improved throughout the study in both groups (Table IV). There were no significant differences in change scores for secondary measures of activity/participation (ArmA, gait speed, Global Assessment Scale, SRB) at any time point. There were no differences in secondary outcomes for participants receiving UL or LL injections in either group.
|
Table IV. Summary of analysis of outcomes of high intensity rehabilitation programmes and usual care: change scores from baseline at 6,12 and 24 weeks |
||||||||
|
6 weeks |
12 weeks |
24 weeks |
||||||
|
High intensity (n = 28) |
Usual care (n = 31) |
High intensity (n = 28) |
Usual care (n = 31) |
High intensity (n = 28) |
Usual care (n = 31) |
|||
|
Primary outcomes |
||||||||
|
Participants achieving ≥ 50% of goals, n (%) |
19 (67.9) |
20 (64.5) |
21 (75.0) |
24 (77.4) |
22 (78.6) |
19 (61.3) |
||
|
GAS T-score, median (IQR) |
12.9 (7.7 to 19.9) |
12.4 (4.6 to 9.1) |
13.4 (11.6 to 25.6) |
15.4 (6.7 to 20.5) |
20.1 (10.4 to 25.5) |
12.3 (5.8 to 18.7) |
||
|
Secondary outcomes |
||||||||
|
MAS, mean (SD) UL & LL UL (n = 40) LL (n = 37) |
–1.0 (0.7) –1.2 (0.7)* –0.7 (0.7) |
–0.7 (0.6) –0.6 (0.5) –0.7 (0.7) |
–0.6 (0.6)* –0.8 (0.7)* –0.4 (0.5) |
–0.3 (0.5) –0.3 (0.7) –0.3 (0.6) |
–0.1 (0.7) –0.4 (0.7) 0.1 (0.6) |
–0.3 (0.4) –0.2 (0.7) –0.5 (0.6)* |
||
|
ArmA, median (IQR) |
n = 21 |
n = 19 |
n = 21 |
n = 19 |
n = 21 |
n = 19 |
||
|
Section A Section B |
–4 (–6 to 0) –2 (–5 to 0) |
–2 (–5.5 to 0) –1 (–2.5 to –0.5) |
–3 (–6 to –1) –2 (–5 to 0) |
–2 (–4– to 1) –1 (–1 to 1) |
–4 (–5 to –1) 0 (–5 to 1) |
–2 (–7 to 1) 0 (–3 to 1) |
||
|
Gait speeda, m/s, median (IQR) |
n = 16 |
n = 20 |
n = 16 |
n = 20 |
n = 16 |
n = 20 |
||
|
0.03 (–0.01 to 0.16) |
0.06 (0.00 to 0.10) |
0.05 (0.01 to 0.10) |
0.03 (0.01 to 0.08) |
0.04 (–0.01 to 0.11) |
0.02 (–0.02 to 0.12) |
|||
|
Global Assessment Scale, median (IQR) |
2 (1 to 3) |
2 (2 to 3) |
2 (2 to 3) |
2 (1 to 3) |
2 (1 to 3) |
2 (1 to 3) |
||
|
SRB |
n = 21 |
n = 26 |
n = 21 |
n = 26 |
n = 21 |
n = 26 |
||
|
Median (IQR) |
0 (–10 to 0) |
–10 (–20 to 0) |
–10 (–20 to 0) |
–10 (–20 to 0) |
–10 (–20 to 10) |
0 (–10 to 10) |
||
|
*p < 0.05. aGait speed (m/s) for participants receiving LL injections. GAS: goal attainment scaling; MAS: Modified Ashworth Scale (change in mean score for treated muscle groups in limb(s)); SD: standard deviation; UL: upper limb; LL: lower limb; ArmA: arm activity measure, section A ‘passive’ and section B ‘active’ arm function (for participants receiving UL injections); SRB: self rated burden (caregivers); IQR: interquartile range. |
||||||||
Factors contributing to effect size
Gender, time since stroke and stroke localization (cortical vs sub-cortical) did not correlate with goal achievement outcomes. Older participants had less change in GAS T-score at 12 weeks (β = –0.27, 95% confidence interval (CI): –0.45 to –0.08, p = 0.005) and 24 weeks (β = –0.26, 95% CI: –0.45 to –0.07, p = 0.009). Participants who achieved at least 50% of their goals at 6 weeks were more likely to do so at 12 weeks (crude OR = 3.7, 95% CI: 1.1–12.8, p = 0.041, adjusted OR = 4.1, 95% CI: 1.1–15.6, p = 0.040) and 24 weeks (crude OR = 5.6, 95% CI: 1.7–18.6, p = 0.005, adjusted OR=6.4 (95% CI: 1.7–23.9, p = 0.005). At 6 weeks, the chance of achieving at least 50% of goals was 3.5 times higher with 1 point decrease in MAS score (95% CI: 1.2–8.9, p = 0.03). However, this correlation was not found at other time points.
Discussion
In this pragmatic controlled trial, a high intensity ambulatory rehabilitation program (mean 3.2 × 1-h sessions per week for approximately 10 weeks) following BoNT-A injections for post-stroke upper and/or lower limb spasticity was compared against a lower intensity, usual care program (mean 1.2 × 1-h weekly sessions). Both groups improved significantly in terms of goal achievement and participant satisfaction up to 24 weeks. There was a strong trend towards UL-injected participants in the high intensity therapy group achieving more goals at 24 weeks compared with usual care. This effect was not observed for LL goals. Demographic and clinical characteristics of study participants were similar to those of other studies including: age (6, 8, 9), time following stroke (11), and proportion of male participants (72% compared with 58–63%) (6, 7, 9, 10). Although the usual care group had more cortical strokes, there were no differences in cortical and cognitive deficits on assessment, and regression analysis did not show any effect of this variable on outcomes. Lower baseline GAS T-scores in the high intensity group implies participants started at a lower level. Although gender, time since stroke and stroke localization (cortical versus sub-cortical) did not correlate with goal achievement outcomes, older participants showed reduced benefit at 12 weeks.
Benefits relating to goal achievement were maintained up to 24 weeks post-injection, even after cessation of therapy and despite the effects of BoNT-A on spasticity wearing off. Other studies have demonstrated a similar delay between spasticity reduction and improved upper limb function (5, 12), suggesting that motor relearning continues after muscle tone is returning to baseline, and supporting the need for BoNT-A to be used with active rehabilitation (12). Most trials of physical interventions or single treatment cycles of BoNT-A for spasticity rarely extend beyond 4 months (23, 34). The longer follow-up in this study ensured that the benefits of therapy and their maintenance after completion of the intervention could be identified. At 6 weeks, spasticity reduction was related to a greater chance of achieving at least 50% of goals, however, this relationship did not continue after 6 weeks. Hence, the prolonged benefits of combined BoNT-A and therapy are more likely to result from other factors such as ongoing neuroplasticity; particularly as the study cohort were a median of 2.5 years post-stroke. This finding supports other literature on chronic (> 1 year) stroke, where long-term motor improvement after participation in novel rehabilitation protocols or therapy modalities were observed due to neuromuscular adaptive changes (26).
This study showed a statistical trend suggesting a benefit of goal-directed higher intensity therapy over usual care following BoNT-A injections to the upper limb. Comparison of the study findings with other studies is limited due to the lack of literature investigating the influence of therapy intensity on outcomes after BoNT-A injections for post-stroke spasticity. Instead, studies have tended to compare single treatment modalities such as stretching, taping or electrical stimulation (17, 18), or qualitatively different therapy approaches such as CIMT and neurodevelopmental therapy (20). Whilst the optimal type and amount of therapy has not been determined, rehabilitation programmes have been suggested to play an important role in improving outcomes following BoNT-A treatment (20, 35, 36). A feasibility study showed that neurological patients receiving therapy (serial casting and movement training) with or without BoNT-A, vs BoNT-A alone, had greater improvement in GAS scores (35). A limited impact of higher intensity rehabilitation programmes (inpatient and outpatient) on functional outcomes after stroke has been found in other studies (37–39), partially supporting the findings of this study. As both study groups received individualized, ambulatory rehabilitation programmes (neurodevelopmental therapy) in similar settings, the current study suggests that while therapy is important, more intensive therapy in late stroke may have a differential capacity to modulate patient outcomes following upper and lower limb BoNT-A injections. However, the ‘black box’ of therapy requires further investigation as spasticity management guidelines lack details on optimal therapy approaches (15, 16). In particular, it may be that provision of goal-directed therapy is more important than the amount of therapy, although further exploration is required.
Limitations of research design have the potential to contribute to the findings of this study. Both groups received active rehabilitation following BoNT-A injections, so there may have been insufficient variation of intensity of therapy. However, the high intensity group received almost 3 times as many therapy sessions compared with the usual care group. In comparison, other studies have reported an intensity differential of only 1.5–2 times the control intervention (37, 39). As the provision of a rehabilitation program in conjunction with BoNT-A injections is considered best practice (15, 16), it was not ethically possible to have a non-therapy control group. More than 50% of participants were receiving therapy at the time of recruitment, which may have influenced the results as some of the benefit of rehabilitation may have already been realized. Furthermore, participants may have been undertaking “informal therapy”, particularly for the lower limb, in the form of walking that may have been much greater in extent than the difference between formal therapy. The different locations of therapy provision between the groups may have been a confounder, as therapy approaches may have differed between sites.
Other factors external to therapy intensity may have impacted on the outcomes of this study. At the participant level, these include their activity levels outside of therapy, personal factors such as motivation and self-efficacy, and participation in study assessments (38) including formal goal setting procedures. At the therapist level, potential confounders include those factors that influence the patient-therapist interaction, compliance, and delivery of therapy.
This trial, conducted in the ‘real-life’ clinical setting, highlights the challenges of investigating complex rehabilitation interventions. A randomized controlled trial design was not possible due to the nature of service delivery, limitations in resources, and those reasons outlined above. Whilst the outcome assessor was blinded, it was not possible to blind therapists. Participants were not informed of study design related to intensity of therapy, however, it is unclear whether this was maintained throughout the study period. Demonstrating functional benefits following focal spasticity management can be difficult as standardized outcome measures are often insensitive (40), particularly with a small sample size and heterogeneous stroke population as in this study. Measurement of goal attainment using GAS sought to overcome some of these issues. GAS assessment has greater responsivity to the effects of BoNT-A than other standardized person-centered or global outcome measures (4–6) and acknowledges the diversity of individualized spasticity management goals. The methodological limitations of this study indicate the need for a larger RCT to establish the role of therapy in achieving patient-centred outcomes after BoNT-A treatment and determining which patients benefit from rehabilitation, particularly comparing upper and lower limb outcomes.
In this study, a statistical trend towards better patient-centred outcomes was observed for the attainment of upper limb goals following more intensive ambulatory rehabilitation following BoNT-A injections for spasticity management. Therapy intensity did not modify post-injection outcomes for the lower limb. This finding leaves open the role of therapy intensity as a post-injection modifier of the ‘black box’ of rehabilitation, at least for the upper limb. Furthermore, overall goal achievement was maintained up to 24 weeks post-injection, even after cessation of therapy and despite the effects of BoNT-A on spasticity wearing off. Despite the lack of literature on the optimal therapy protocols, therapy is routinely provided following BoNT-A injections at a significant cost to health care services. In light of this and the study findings, further research to determine the optimal types, intensities and timing of multidisciplinary therapy programmes following BoNT-A injections and ideal patient selection is warranted.
Acknowledgements
We are grateful to all participants and therapists who provided therapy. We thank the Rehabilitation Medicine Department staff, including Drs: Geoff Abbott, Louisa Ng, Edwin Luk, Ishani Rajapaksa, Aarathi Vaska and Brinda Thirugnanam; Shane McSweeney for performing outcome assessments, and Lynne Turner-Stokes for providing valuable advice. This study was partially funded by the Australasian Faculty of Rehabilitation Medicine, Ipsen Open Research Fellowship. The funder has had no influence on the interpretation of data and the final conclusions drawn.
Conflict of interest
Marina Demetrios is on the Advisory Board for Ipsen and has received sponsorship to attend meetings from makers of BoNT-A (Allergan and Ipsen). Ian Baguley has received honoraria, sponsorship to attend meetings and/or consultancy fees from Allergan and Ipsen. Julie Louie has received honoraria from Ipsen. No author has a personal financial interest in BoNT-A or in any of the methods used in this research.
References
