Ingrid Brands, MD1, Sebastian Köhler, PhD2, Sven Stapert, PhD3, Derick Wade, MD PhD4,5 and Caroline van Heugten, PhD2,3
From the 1Department of Neurorehabilitation, Libra Rehabilitation Medicine & Audiology, Eindhoven, 2School for Mental Health and Neuroscience, Alzheimer Centre Limburg, Faculty of Health, Medicine and Life Sciences, 3Department of Neuropsychology and Psychopharmacology, Faculty of Psychology and Neuroscience, 4Department of Rehabilitation Medicine, School for Public Health and Primary Care, Maastricht University, Maastricht, The Netherlands and 5Oxford Centre for Enablement, Oxford, UK
OBJECTIVES: To investigate coping flexibility in patients with newly acquired brain injury and to investigate the influence of problem type, self-efficacy, self-awareness and self-reported executive functions on coping flexibility.
METHODS: Data were collected from a prospective clinical cohort study of 136 patients assessed after discharge home (mean time since injury = 15 weeks) and 1 year later. Situation-specific coping was measured by asking patients to complete the Coping Inventory for Stressful Situations (CISS) for 3 acquired brain injury (ABI)-related situations, which were then categorized into problem types (physical, cognitive, emotional, behavioural, communication, other). Coping consistency (number of strategies used throughout every situation) and variability (range in frequency of use of strategies over situations) were measured. Random effects regression analyses were used.
RESULTS: Patients used more task-oriented coping for cognitive compared with physical problems. Coping variability was limited. Reliance on emotion-oriented coping decreased over time. Higher self-efficacy correlated with increased task-oriented and avoidance coping and decreased emotion-oriented coping. Greater self-reported problems in executive function correlated with greater consistency in task-oriented and emotion-oriented strategies.
CONCLUSION: Patients with acquired brain injury rely on a defined set of coping options across situations and time. High self-efficacy increases active coping. Subjective executive dysfunction might hamper effective strategy selection.
Key words: coping skills; adaptation; self-efficacy; awareness; executive function; brain injury.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Ingrid Brands, PO Box 1355, NL-5602 BJ Eindhoven, The Netherlands. E-mail: i.brands@libranet.nl
Accepted Apr 3, 2014; Epub ahead of print Jun 19, 2014
Introduction
Coping, the cognitive and behavioural efforts made to manage stressful situations (1), is considered to be an important determinant of psychosocial adaptation after acquired brain injury (ABI). The use of a few specific coping styles, such as passive coping and coping characterized by wishful thinking, avoidance, worry, self-blame and substance abuse, have been consistently associated with poor quality of life (2, 3). High use of escape-avoidance coping has a negative effect on return to productivity (4). Poor executive functioning is the best predictor of escape-avoidant coping (5).
Furthermore, high self-efficacy has been associated with better psychosocial adjustment and quality of life in traumatic brain injury (TBI) (6). Self-efficacy refers to the belief in one’s capabilities in achieving goals (7). Self-efficacy beliefs are domain-specific, but various and numerous experiences of success and failure in different domains of functioning may generate a generalized belief of self-efficacy. In the healthy population, individuals with higher self-efficacy displayed higher use of active and problem-focused coping (8). However, it is not known whether the same is true for patients with ABI.
It has been proposed that access to a flexible repertoire of coping strategies is an important determinant of coping effectiveness and well-being in general (9). Coping flexibility means that coping styles can readily be adapted to changing contextual demands (9). Lester et al. (10) have shown, in the healthy population, that individuals who display a greater variability in their coping responses to different situations report greater well-being. Others have found that healthy individuals exhibit higher levels of self-reported coping variability than do chronically ill people (11). In patients with rheumatoid arthritis, increased coping flexibility and illness acceptance has been associated with decreased psychological stress (12).
For patients with ABI, coping flexibility may be important, yet it has not been studied intensively. Most studies have used a cross-sectional design and measured coping with stress in general or in one particular stressful situation, with largely inconsistent findings (13–15). For instance, Moore et al. (13) and Moore & Stambrook (14) found that patients with TBI who use high levels of a wide variety of coping styles showed worse psychosocial adjustment than patients characterized by either low use of all coping styles or high use of positive reappraisal and social support seeking. Medley et al. (15) found higher overall strategy use in TBI patients with good self-awareness, high levels of emotional distress and high perceived control than in patients with impaired awareness and lower stress levels.
While studying the overall use of coping styles can identify inter-individual differences in the amount and patterns of strategy use, it does not provide information on an individual patient’s ability to use different strategies according to changing situational demands, i.e. intra-individual differences. Coping flexibility might be dependent on problem/stressor characteristics (11) and specific brain-injury related symptoms (16), such as dysexecutive functions and impaired self-awareness, resulting in impaired problem-solving and anticipation, decreased mental flexibility and diminished problem perception.
In summary, few studies have investigated coping flexibility in ABI. In ABI, self-efficacy, executive functioning and self-awareness have been shown to influence coping, but their relationship to coping flexibility is unknown.
Coping flexibility has many aspects. The aims of the present study in patients with ABI, were: (i) to explore whether coping responses are adapted across situations, according to the type of problem encountered and over time; (ii) to investigate coping variability (the range in frequency of use of strategies across situations) and coping consistency (one’s fixed situation-independent coping repertoire) and its evolution over time; and (iii) to investigate the influence of self-reported executive functioning, self-awareness and self-efficacy on coping, variability and consistency. We expected to find differences in coping responses based on problem characteristics. We furthermore hypothesized that: (i) high self-efficacy is associated with higher use of problem-focused coping and lower coping consistency; (ii) impaired self-awareness is associated with low overall use of coping and low variability; and (iii) self-reported problems in executive functions are associated with greater coping consistency.
MethodS
Patients
Between January 2011 and January 2012, rehabilitation physicians and neurologists of the participating institutes (2 rehabilitation centres and 2 hospitals in the south of the Netherlands) recruited patients who were eligible for participation in this prospective clinical cohort study and, if available, their significant others (spouse, adult child, sibling, parent). Patients were included consecutively upon return to the home environment, either at the start of outpatient neurorehabilitation or at discharge home from hospital or from inpatient neurorehabilitation. The inclusion criteria were: (i) age ≥ 18 years; (ii) newly acquired, non-progressive brain injury of any aetiology confirmed by neurological and/or neuroimaging data; (iii) for patients recruited at the start of outpatient rehabilitation maximum time since injury 4 months. Exclusion criteria were: (i) any premorbid progressive brain disease; (ii) insufficient command of the Dutch language; (iii) inability to complete questionnaires based on clinical judgement (aphasia, severe cognitive impairment).
This study was approved by the medical ethics committees of Maastricht University Medical Centre and all participating hospitals and rehabilitation centres. All patients and significant others provided written informed consent.
Procedure
Baseline assessment consisted of questionnaire assessments and a telephone interview that took place within 6 weeks after inclusion. Patients were interviewed when they had been at home for at least 2–4 weeks. They were asked about the 3 most stressful situations that they had encountered as a consequence of their brain injury during the previous 2 weeks.
The situations mentioned by each participant were categorized into problem types: physical, cognitive, emotional, behavioural, communicative and other. Categorization was done by the research assistant and verified by the first author. For example, the situation “I am very stressed when I meet people because I cannot remember their names” was classified as a cognitive problem. For each of the 3 stressful situations, a separate coping questionnaire (CISS, see below) was prepared in which the instruction for completion was made specific: “How much do you engage in these types of activities when you are confronted with… (1 of 3 situations)?”.
Each patient received 3 situation-specific coping questionnaires (CISS) by post, together with questionnaires about self-efficacy, self-awareness, executive functioning and emotional distress. The significant other received the “other” version of the awareness questionnaire by post.
Follow-up assessment took place 1 year (±4 weeks) after inclusion. Interviews and questionnaire assessments were repeated. The patient again identified 3 brain-injury related stressful situations at that time.
If a patient preferred a live interview or needed assistance in completing the questionnaires, a face-to-face interview with the research assistant or first author was arranged (n = 19, 14% of the cases at baseline; n = 18, 15% of the cases at follow-up).
For this study, from the patients who consented (148 out of 190 patients who were asked to participate), we only used the data of those who completed the coping questionnaires for all 3 situations.
Measurements
Coping. Basic coping strategies were measured using the Coping Inventory for Stressful Situations (CISS) (17, 18). The 48-item CISS has 3 scales (16 items per scale): task-oriented (T), emotion-oriented (E) and avoidance (A). The avoidance scale contains 2 subscales: social diversion (SD) and distraction (D). Item scores (1 = not at all, to 5 = very much) are summed per scale; higher scores indicate a greater use of that particular coping style.
Coping variability and consistency. Coping variability reflects the range in frequency of use of strategies over situations (10). For each CISS item, over the 3 CISS questionnaires, the score range was calculated by extracting the lowest score of the 3 lists for that particular item from the highest (score range maximum = 4, minimum = 0) (Fig. 1). The mean of all item score ranges per scale formed CISS scale score ranges.
Coping consistency reflects one’s fixed coping repertoire, the strategies used invariably throughout every situation (19). It is non-judgemental and can refer to desirable (e.g. stable, resilient and systematic) as well as undesirable (e.g. invariable, inflexible and rigid) coping behaviour. To quantify consistency, we counted for each CISS scale (T, E, A) how many of its 16 CISS items (= coping strategies) were used in all 3 situation-specific CISS questionnaires (item score ≥ 2 in each questionnaire) (Fig. 1). This resulted in CISS scale fixed scores (maximum = 16, minimum = 0). Higher CISS scale fixed scores mean that more coping strategies of the respective scales (T, E, A) are used throughout every situation.
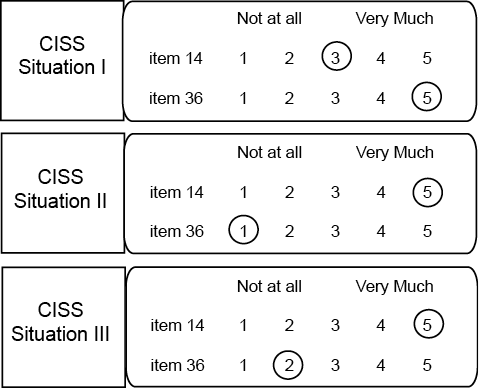
Fig. 1. Coping Inventory for Stressful Situations (CISS) questionnaire (2 out of 48 items are selected). The instruction for completion is: “How much do you engage in these types of activities when you are confronted with… (1 of 3 stressful situations)?”.
Example of calculation of item score ranges for a hypothetical subject:
Item 14 score range: 5 (highest score) – 3 (lowest score) = 2.
Item 36 score range: 5 (highest score) – 1 (lowest score) = 4.
Example of counting scale fixed scores for a hypothetical subject:
Item 14: score ≥ 2 in all 3 CISS questionnaires, thus item 14 is used in every situation and contributes to CISS-E fixed score.
Item 36: score in CISS II questionnaire = 1 (not at all), so this strategy is not used in every situation and thus item 36 does not contribute to CISS-T fixed score.
Self-efficacy. To measure self-efficacy for managing brain-injury specific symptoms, we used the 13-item TBI Self-efficacy Questionnaire (SEsx) (6), which contains 4 subscales: social (4 items), physical (1 item), cognitive (4 items) and emotional (4 items) measuring self-efficacy for obtaining help from community, family and friends to perform everyday activities and obtain emotional support, managing physical symptoms, managing and compensating for cognitive symptoms, and managing emotional symptoms (e.g. feeling frustrated or overwhelmed), respectively. Items scores (1 = not at all confident, to 10 = totally confident) are summed per subscale. Subscale scores are summed to a total score. Higher scores indicate greater self-efficacy.
To measure general self-efficacy, we used the 12-item Dutch version (20) of the original General Self-efficacy Scale (GSES) (21). Items are scored on a 5-point Likert scale. Higher scores indicate higher general self-efficacy (maximum score = 60).
Brain-injury specific symptoms. Self-awareness was measured with the 17-item Awareness Questionnaire (AQ), which assesses how well individuals perform on a variety of activities compared with before their injury (1 = much worse, to 5 = much better). All item scores are summed. The AQ consists of “self” and “other” versions completed by the participant and family member or significant other. Self-awareness is calculated by subtracting others’ rating from the patients’ own rating, resulting in a discrepancy score. Positive discrepancy scores indicate that patients underestimate their deficits; higher scores are associated with greater degrees of impaired self-awareness (22, 23).
The patient’s subjective executive functioning was measured with the 20-item self-rating form of the Dysexecutive Questionnaire (DEX-P) (24). Items are rated on a 5-point Likert scale indicating frequency of occurrence of symptoms (0 = never, to 4 = very often). Higher patient scores indicate a higher level of self-perceived executive problems.
Other measures. The Hospital Anxiety and Depression Scale (HADS) was used as a general measure of emotional distress. The HADS contains 14 items, scored on a 4-point scale (range 0–3), which form 2 subscales: anxiety and depression (25). HADS subscale scores ≥8 might indicate the presence of depression or anxiety.
Demographic data and lesion characteristics (gender; age; date of brain injury; type of lesion) were collected from the medical files. The level of educational attainment was recorded during baseline interview and classified according to a 3-level system often used in the Netherlands: primary education (low), junior vocational training (medium) and senior vocational or academic training (high), corresponding to 8.6 (SD 1.9), 11.4 (SD 2.5), and 15.2 (SD 3.3) years of full-time education respectively (26).
Statistical analyses
Independent t-tests and χ2 tests were calculated to detect differences in demographic and clinical characteristics between study completers and patients lost to observation. Paired-samples t-tests were used to compare baseline and follow-up scores of all clinical variables.
A random effects (mixed model) regression analysis was used to test whether coping styles (CISS scale scores: T, E, A) differed across situations, over time and according to the type of problem (physical, cognitive, emotional, behavioural, communicative and other). The analyses account for the fact that repeated measures were taken from individuals when asking patients to complete the CISS for 3 different situations and at 2 time-points (baseline, 1 year follow-up). Thus, analyses made full use of all available data, but were adjusted for the fact that repeated measures are correlated within individuals, yielding pooled effect estimates. We also tested whether estimates were further influenced by demographic (age, gender, education) and clinical variables (self-efficacy, self-awareness, self-reported executive functioning, emotional distress).
Model development consisted of several steps. First, all demographic variables and the variables denoting situation (situation 1, 2, 3) and time (baseline, follow-up) were entered. Then the associations with clinical variables (GSES, SEsx, DEX-P, AQ discrepancy, HADS) were tested separately, and those who showed associations at a p-value < 0.10 were retained. Finally, any clinical variable that was not statistically significant at a p-value of < 0.05 in the full multivariable model was manually removed in a backward selection procedure, thus starting with the variables with the highest p-value.
To investigate the influence of demographic and clinical variables on coping variability and consistency, the analyses noted above were repeated using the CISS scale score ranges (variability) and CISS scale fixed scores (consistency) as dependent variables in the random effects regression analyses. All analyses were carried out in Stata 12.1 (StataCorp LP, TX, USA), using 2-sided hypothesis testing with an alpha-level of 0.05.
Results
At baseline (mean time since injury 15.3 weeks) our study sample consisted of 136 patients of whom 118 (87%) completed all 3 CISS questionnaires at follow-up (mean time since injury 66.8 weeks). Patients lost to observation (13%) did not differ significantly in baseline coping or in any of the demographic and clinical variables. Table I shows the demographic and injury-related characteristics of the study sample. Description of all clinical variables is summarized in Table II.
|
Table I. Patient characteristics at baseline (n = 136) |
|
|
Patient characteristics |
|
|
Age, years, mean (SD) |
56 (11.7) |
|
Time since injury, weeks, mean (SD) |
15.3 (8.6) |
|
Gender, male, n (%) |
88 (64.7) |
|
Educational level, n (%) |
|
|
Low |
39 (28.7) |
|
Medium |
54 (39.7) |
|
High |
43 (31.6) |
|
Type of lesion, n (%) |
|
|
Infarction |
89 (65.4) |
|
SAH |
10 (7.4) |
|
ICH |
9 (6.6) |
|
Diffuse vascular lesions |
2 (1.5) |
|
TBI |
11 (8.1) |
|
Anoxic encephalopathy |
3 (2.2) |
|
Tumour benign |
5 (3.6) |
|
Menigitis/encephalitis |
1 (0.7) |
|
Other |
6 (4.4) |
|
SD: standard deviation; SAH: subarachnoid haemorrhage; ICH: intracerebral haemorrhage; TBI: traumatic brain injury. |
|
|
Table II. Descriptive data of all clinical variables and distribution of positive Awareness Questionnaire (AQ) discrepancy scores, indicating underestimation of deficits by the patient |
|||||||||||
|
Baseline |
One-year follow-up |
t |
p |
||||||||
|
n |
Mean (SD) |
Median (IQR) |
n |
Mean (SD) |
Median (IQR) |
||||||
|
GSES |
135 |
44.82 (9.95) |
45 (36–53) |
118 |
43.80 (10.07) |
44 (37–53) |
0.95a |
0.34 |
|||
|
SEsx soc |
136 |
27.24 (7.03) |
27 (24–32) |
118 |
28.14 (6.72) |
29 (25–32) |
−1.57b |
0.12 |
|||
|
SEsx phys |
136 |
5.95 (2.38) |
6 (4–8) |
118 |
6.23 (2.36) |
6.5 (4–8) |
−1.24b |
0.22 |
|||
|
SEsx cogn |
136 |
24.32 (8.75) |
24 (18.3–32) |
118 |
26.03 (8.40) |
27 (20–32) |
–2.96b |
0.004 |
|||
|
SEsx emot |
136 |
25.54 (7.88) |
26 (20–32) |
118 |
27.20 (7.56) |
28 (22–33) |
−2.99b |
0.003 |
|||
|
SEsx |
136 |
83.04 (22.85) |
81 (67.3–102.8) |
118 |
87.60 (21.79) |
87 (72–104) |
−2.89b |
0.005 |
|||
|
DEX-P |
134 |
21.79 (14.47) |
20 (10.8–31) |
118 |
22.59 (14.67) |
19.5 (11–32) |
−0.17a |
0.86 |
|||
|
HADS-A |
134 |
6.09 (3.99) |
6 (3–9) |
118 |
5.41 (3.87) |
5 (2–8.3) |
2.53c |
0.01 |
|||
|
HADS-D |
134 |
5.97 (4.24) |
6 (2–9) |
118 |
5.37 (4.44) |
4 (2–8.3) |
2.27c |
0.03 |
|||
|
HADS |
134 |
12.10 (7.40) |
11 (6–17) |
118 |
10.78 (7.59) |
9 (4.8–17) |
2.76c |
0.007 |
|||
|
n |
% |
n |
% |
||||||||
|
AQ discr 1–9 |
51 |
41 |
39 |
35 |
|||||||
|
AQ discr 10–19 |
7 |
6 |
8 |
7 |
|||||||
|
AQ discr 20–29 |
0 |
0 |
1 |
1 |
|||||||
|
AQ discr > 29 |
0 |
0 |
1 |
1 |
|||||||
|
adf = 116; bdf = 117; cdf = 115. SD: standard deviation; GSES: General Self-efficacy Scale; SEsx soc; Social Subscale of TBI Self-efficacy Questionnaire; SEsx phys: Physical Subscale of TBI Self-efficacy Questionnaire; SEsx cogn: Cognitive Subscale of TBI Self-efficacy Questionnaire; SEsx emot: Emotional Subscale of TBI Self-efficacy Questionnaire; SEsx: TBI Self-efficacy Questionnaire total score; DEX-P: Dysexecutive Questionnaire patient form; HADS-A: Anxiety Subscale of Hospital Anxiety and Depression Scale; HADS-D: Depression Subscale of Hospital Anxiety and Depression Scale; HADS: Hospital Anxiety and Depression Scale total score; AQ discr: Awareness Questionnaire discrepancy score; df: degrees of freedom. |
|||||||||||
HADS-D scores were above cut-off indicative for depression in 37% of patients at baseline and in 32% at follow-up. For HADS-A, at both measurements 32% of patients scored above cut-off indicative for anxiety. The types of problem that were mentioned most frequently as being highly stressful were physical and cognitive problems (Table III).
|
Table III. Problem types, defined to categorize the stressful situations patients encountered due to their brain injury |
||
|
Type of problem |
Baseline n (%) |
One-year follow-up n (%) |
|
Physical |
132 (32.4) |
111 (31.4) |
|
Cognitive |
161 (39.5) |
171 (48.3) |
|
Emotional |
18 (4.4) |
24 (6.8) |
|
Behavioural |
16 (3.9) |
6 (1.7) |
|
Communication |
42 (10.3) |
31 (8.8) |
|
Other |
39 (9.6) |
11 (3.1) |
|
Total problems |
408 (100.0) |
354 (100.0) |
In Table IV, coping variability and consistency scores are presented. CISS scale score ranges (variability) were small (<1.0) for both measurements. CISS scale fixed scores (consistency) were highest for task-oriented coping and lowest for emotion-oriented coping.
|
Table IV. Coping Inventory for Stressful Situations (CISS) scale score ranges and CISS scale fixed scores |
||
|
Baseline Mean (SD) |
One year follow-up Mean (SD) |
|
|
Range CISS-T |
0.95 (0.52) |
0.96 (0.54) |
|
Range CISS-E |
0.80 (0.51) |
0.81 (0.52) |
|
Range CISS-A |
0.73 (0.50) |
0.77 (0.47) |
|
Fixed CISS-T |
12.5 (4.1) |
12.3 (4.6) |
|
Fixed CISS-E |
7.4 (5.2) |
6.6 (5.1) |
|
Fixed CISS-A |
9.2 (5.1) |
8.8 (5.1) |
|
SD: standard deviation; CISS-T: Task scale of the Coping Inventory for Stressful Situations (CISS); CISS-E: CISS Emotion scale; CISS-A: CISS Avoidance scale. |
||
Task-oriented coping
CISS-T scores did not differ significantly over time or over situations. However, patients used more task-oriented coping when faced with cognitive problems than with physical problems (b = 1.55, 95% confidence interval (CI) 0.50–2.61, p = 0.004). From the clinical variables, only GSES scores were significantly associated with CISS-T scores (b = 0.24, 95% CI 0.10–0.38, p = 0.001), indicating that reliance on task-oriented coping increased with increasing general self-efficacy. However, differences in self-efficacy did not explain the difference among task-oriented coping with physical vs cognitive problems.
For CISS-T score range, only a significant effect of age was observed (b = −0.008, 95% CI −0.01 to −0.002, p = 0.013), indicating that the variability in the use of task coping decreased modestly with increasing age.
For CISS-T fixed scores, the final model showed a significant effect for DEX-P scores only (b = 0.04, 95% CI 0.0003–0.08, p = 0.048), indicating that the number of task-oriented strategies used consistently in all 3 situations increased with increasing self-reported executive problems.
Emotion-oriented coping
CISS-E scores decreased over time (b = −2.30, 95% CI −4.12 to −0.48, p = 0.013). Emotion-oriented coping appeared to be used more often when dealing with behavioural problems compared with physical problems, though differences did not reach statistical significance (b = 1.99, 95% CI −0.44 to 4.42, p = 0.11). Adding the clinical variables, SEsx scores (b = −0.10, 95% CI −0.17 to −0.03, p = 0.004), DEX-P scores (b = 0.21, 95% CI 0.10–0.31, p < 0.001) and HADS scores (b = 0.56, 95% CI 0.34–0.77, p < 0.001) were significantly associated with CISS-E scores, indicating that reliance on emotion-oriented coping decreased with increasing self-efficacy for the management of brain-injury related symptoms and increased with higher levels of emotional distress and self-reported executive problems. CISS-E scores showed a significant association with level of education (high vs low: b = −7.99, 95% CI −12.60 to −3.38, p = 0.001), indicating that highly educated people made considerably less use of emotion-oriented coping.
CISS-E score range only showed a significant association with SEsx scores (b = −0.008, 95% CI −0.01 to −0.005, p < 0.001), indicating that variability in emotion-oriented coping decreased with increasing self-efficacy for managing brain-injury related symptoms.
CISS-E fixed scores decreased over time (b = −0.98, 95% CI −1.81 to −0.15, p = 0.02). For the clinical variables, a significant effect of DEX-P scores (b = 0.09, 95% CI 0.04–0.14, p = 0.001) and HADS scores (b = 0.24, 95% CI 0.15–0.34, p<0.001) was observed. CISS-E fixed scores also showed a significant dose-response effect with level of education (medium vs low: b = −1.92, 95% CI −3.79 to −0.05, p = 0.04, high vs low: b = −3.04, 95% CI −5.00 to −1.10, p = 0.002). Therefore, the number of emotion-oriented strategies used throughout every situation increased with increasing self-reported executive dysfunction and higher emotional distress and decreased with higher education.
Avoidance coping
CISS-A scores did not differ significantly over time, but showed a significant difference over situations (situation 2 vs 1: b = −1.21, p = 0.002; situation 3 vs 1: b = −1.24, p = 0.007), indicating intra-individual variability. There was no effect for problem category. Adding the clinical variables, only GSES scores showed a significant association with CISS-A scores (b = 0.20, 95% CI 0.06–0.35, p = 0.006), indicating that the use of avoidance coping increased with increasing general self-efficacy. A significant association with education (high vs low: b = −7.36, 95% CI −11.75 to −2.96, p = 0.001) indicated that, with higher education, the use of avoidance coping decreased substantially.
No significant effects were found for CISS-A score range.
For CISS-A fixed scores, a significant association was observed for level of education only (high vs low: b = −3,34, 95% CI −5.24 to −1.43, p = 0.001), indicating lower consistent reliance on avoidance strategies with higher education.
Discussion
In this prospective study of a large group of patients over a period of 1 year after acute disabling brain injury, we tested whether they adapted their coping responses over situations and according to the type of problem encountered. We explored coping variability and consistency and examined whether coping style, variability and consistency were dependent on clinical characteristics.
We found that patients relied on a limited set of coping strategies, both over time and across different situations. Contrary to our expectations, the type of problem denoted as most stressful was of limited influence on patients’ use of coping styles. Patients only used more task-oriented coping when confronted with cognitive problems compared with physical problems.
In line with previous studies, cognitive problems were the most frequently mentioned cause of major distress (27). However, the association between behavioural problems and emotion-oriented coping style seems to deserve further research as our analyses were probably underpowered (only 4% of patients reported behavioural problems). Yet, in patients diagnosed with multiple sclerosis, rheumatoid arthritis or systemic lupus erythematosus, coping responses were not influenced by specific stressor characteristics (11).
Overall, coping variability (CISS scale score ranges varied from 0.7 to 0.9) was small, indicating that patients generally do not make major switches in the frequency of use of strategies over different situations (e.g. from very limited to extensive use). Consistency for task-oriented coping was high (12 of 16 strategies were used in every situation), which is in line with the findings of Kendall et al. (19).
Over time, only the use of emotion-oriented coping changed, and it decreased.
Higher self-efficacy in managing brain injury-specific symptoms correlated with lower reliance on emotion-oriented coping and a lower variability in use of emotion-oriented strategies. This stress-buffering effect of self-efficacy is well documented in healthy people (28) and cancer patients (29). In addition, the association between low self-efficacy and increased use of emotion-oriented coping has been reported in other chronic diseases (30, 31).
Moreover, as we expected, we found that higher general self-efficacy was associated with higher use of task and avoidance coping. At first glance, the latter association might seem odd, but it must be noted that the CISS avoidance scale contains many items referring to actively seeking social support and distraction, whereas in other coping questionnaires avoidance is frequently associated with a passive, internalizing attitude. Our results suggest that self-efficacy facilitates the use of both restoration-oriented behaviour (task coping) and loss-oriented coping (avoidance). Achieving maximal restoration of function and adjusting to alterations and losses are simultaneous processes involved in the adaptation process after brain injury (32). The association between active coping and high self-efficacy has been documented in chronically ill people (33) and in the healthy population (34).
Patients reporting greater executive dysfunction used more emotion-oriented coping. Furthermore, in accordance with our hypothesis, greater self-reported executive problems correlated with higher coping consistency for task-oriented and emotion-oriented coping. The invariable deployment of a greater amount of task and emotion strategies throughout situations might reflect problems in effective strategy selection.
A low level of education was consistently related to higher reliance on emotion-oriented and avoidance coping and to higher consistency in use of both styles, mirroring earlier studies showing associations between non-productive coping (2), social-emotional coping (35) and low level of education.
Our findings may have implications for clinical practice. To optimize adjustment to the diverse consequences of ABI, we suggest training patients, especially those who experience problems in executive functioning, to use a small, but effective, set of coping strategies. Furthermore, these findings suggest that therapeutic attempts to enhance self-efficacy during rehabilitation would be appropriate, as this seems to promote active coping. Cognitive behavioural therapy has shown to be effective in increasing the ability to implement adaptive coping strategies (36) and in improving coping self-efficacy (37).
In addition, according to Bandura (7), self-efficacy beliefs are not only developed through direct mastery experiences, but also by vicarious experiences, verbal persuasions and changes in physiological and affective states from which people judge their capabilities. When training skills and strategies, one could think of adding elements such as referential comparison with significant others, positive feedback, reward and providing control over interfering emotions, e.g. stress, anxiety and fatigue, or making use of behavioural experiments.
This study has several strengths. It is the first study to examine coping flexibility related to real life problems in ABI using a prospective design. We took multiple approaches to measure coping flexibility, since there is no standardized method, and in our analyses, we controlled for emotional distress. We did not limit our inclusion criteria to a single diagnosis as it has been demonstrated repeatedly that severity and type of brain injury did not influence coping (2, 38). The fact that our study sample is a mixed composition of patients with ABI due to stroke, TBI, and anoxic encephalopathy, among other causes, enhances the generalizability of our results. Loss to observation was small (13% of cases) and did not lead to selection bias.
The limitations of the present study should also be acknowledged. As we did not compare our results with a healthy control sample, we cannot determine whether coping variability or consistency in ABI differs from the healthy population. We did not formally test the executive performance of the subjects. However, in ABI patients with prominent neuropsychiatric symptoms coping was found to be associated with subjective executive functioning, but not with objective executive performance (39). Despite a relatively large sample size, small subgroup sizes might have influenced detection of associations with less frequently mentioned problem categories and self-awareness. According to the criteria by Sherer et al. (23) only 2% of our patients at follow-up could be categorized as having moderate and severe impaired self-awareness. The small number of TBI patients in our sample (8%) might explain this. In TBI, awareness deficits occur more frequently due to the higher incidence of frontal lobe damage (40). To measure self-efficacy for managing brain injury specific symptoms we used the SEsx, which, to date, is only validated for use in TBI (6).
In conclusion, this study showed that patients with ABI rely on a rather defined set of coping options across situations and time. Coping style is only modestly influenced by problem type. Self-efficacy enhances task and avoidance coping, which are both needed in adapting to the consequences of ABI. Subjective executive dysfunction increases consistency in task and emotion strategy use, which might suggest problems in effective strategy selection and could be a target for rehabilitation therapy.
Acknowledgements
The authors wish to thank John Bouwmans for data collection, and the participating institutions: Blixembosch Rehabilitation Centre, Eindhoven; Leijpark Rehabilitation Centre, Tilburg; Orbis Medical Centre, Sittard; and Atrium Medical Centre, Heerlen.
This work received support from CZ Fonds grant AFVV11-045.
The authors declare no conflicts of interest.
References
