John Robert Bach, MD1, Louis Ralph Saporito, BS1, Harsh Rakesh Shah, BS2 and Diane Sinquee, MD3
From the 1Department of Physical Medicine and Rehabilitation, Rutgers New Jersey Medical School, University Hospital, Newark, NJ, 2New York Institute of Technology-College of Osteopathic Medicine, Old Westbury, NY and 3Department of Pediatrics, Rutgers New Jersey Medical School, University Hospital, Newark, NJ, USA
OBJECTIVE: Ventilator dependent patients with neuromuscular disorders and high level spinal cord injury have been extubated and decanulated to continuous noninvasive intermittent positive pressure ventilatory support after mechanical insufflation-exsufflation was used to achieve specific criteria for tube removal. The purpose of this study is to report changes in extent of need for ventilator use and in vital capacity related to mechanical insufflation-exsufflation used via tracheostomy tubes and post-decanulation via oronasal interfaces.
METHODS: Upon presentation patients were placed on fiO2 21% and CO2 was normalized by adjusting ventilator settings as needed. The vital capacity (1st data point) and h/day of ventilator dependence were noted. Then mechanical insufflation-exsufflation was used via the tubes up to every 2 h until ambient air oxyhemoglobin saturation (SpO2) baseline remained ≥ 95% and other decanulation criteria were achieved. The vital capacity was re-measured (2nd data point) and the patient decanulated to continuous noninvasive intermittent positive pressure ventilatory support in ambient air as care providers used mechanical insufflation-exsufflation up to every 30 min to maintain SpO2 ≥ 95%. The vital capacity (3rd data point) and minimum hours/day of noninvasive intermittent positive pressure ventilatory support requirement during the next 3 weeks were recorded.
RESULTS: The vital capacities of 61 tracheostomized ventilator users, 36 of whom were continuously dependent, increased significantly (p < 0.001) from presentation to immediately pre-decanulation and in the 3 weeks post-decanulation and all except one were successfully decanulated.
CONCLUSION: Many ventilator users can be decanulated in outpatient clinics to continuous noninvasive intermittent positive pressure ventilatory support with mechanical insufflation-exsufflation used to increase vital capacity, SpO2, and autonomous ability to breathe.
Key words: Duchenne muscular dystrophy; noninvasive mechanical ventilation; mechanical ventilation; decanulation; mechanical insufflation-exsufflation; neuromuscular disease; spinal cord injury; respiratory.
J Rehabil Med 2014; 00: 00–00
Correspondence address: John R. Bach MD, Department of Physical Medicine and Rehabilitation, University Hospital B-403, 150 Bergen Street, Newark, NJ 07103, USA. E-mail: bachjr@njms.rutgers.edu
Accepted May 26, 2014; Epub ahead of print Aug 6, 2014
Introduction
In 1990 and 1991 we reported decanulation of 50 patients with high-level spinal cord injuries (SCI). Many who were dependent on continuous tracheostomy mechanical ventilation (CTMV) were decanulated to continuous noninvasive intermittent positive pressure ventilatory support (CNVS) with mechanical insufflation-exsufflation (MIE) used to expel airway secretions and maintain ambient air oxyhemoglobin saturation (SpO2) ≥ 95% (1, 2). Although some have remained CNVS dependent for as long as 23 years (Fig. 1), most whose vital capacities (VCs) subsequently exceeded 250 ml weaned to part-time NVS in the early post-decanulation period by taking fewer and fewer ventilator delivered volumes via angled mouth pieces. Whereas spontaneous improvement and weaning can occur over time for SCI patients (3), it does not occur for patients with progressive neuromuscular disease (NMD). Nevertheless, 6 out of 8 CTMV dependent patients with Duchenne muscular dystrophy (DMD) were decanulated to CNVS and they too weaned to part-time NVS (4).
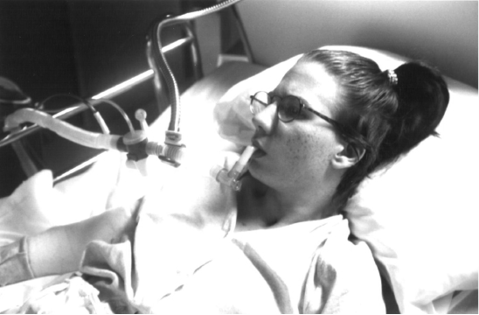
Fig. 1. 17-year-old girl with C2 spinal cord injury in 1992 decanulated to mouth piece continuous noninvasive intermittent positive pressure ventilatory support (CNVS) with 200 ml of vital capacity, currently 38 years of age and still CNVS dependent.
Similarly, in 2010 we reported the successful extubation of 155 of 157 ventilator “unweanable” patients to CNVS despite some having been intubated for up to 80 days and having failed up to 4 extubation attempts prior to transfer to our units (5, 6). Upon presentation for extubation, few patients had SpO2 ≥ 95% in ambient air (5). However, after introduction of MIE most met this milestone within 24 to 48 h (range 0–11 days) and were then extubated to CNVS. With continued use of MIE to maintain normal ambient air SpO2, (5) most patients whose VCs increased to exceed 250 ml also weaned to part-time NVS (Fig. 1). Thus, the paradigm of weaning to extubate or decanulate became extubate or decanulate to permit self-weaning.
In 1954, increases in VC of 15 to 42% were reported for 67 patients with “obstructive dyspnea” due to airway secretions by using MIE (6). In 1993, we reported normalization of SpO2 along with increases in VC of 15% to 400% (mean 55%) after using MIE for patients with NMDs during chest infections (7). The purpose of this study was to describe changes in VC and in hours of daily need for ventilatory support associated with MIE use before and after decanulation of patients with severe respiratory muscle insufficiency (SRMI), most of whom were decanulated in physical medicine and rehabilitation out-patient clinics. Outpatient decanulation of ventilator dependent patients has never before been reported.
Methods
The records of all patients with SRMI who were referred for decanulation from January 1999 through 2013 were reviewed as approved by our Institutional Review Board. Inclusion criteria were dependence on CTMV due to SRMI and desire to be decanulated to NVS. Exclusion criteria were age less than 16 years, persistent medical instability, lack of cooperation, failure to achieve SpO2 ≥ 95% on ambient air despite full ventilatory support and frequent MIE, or maximum cough peak flows (CPF), with or without assistance, less than 120 l/m with CPF between 120 and 160 L/min being a relative contraindication (8). To achieve 160 l/min, the patients were asked to cough via an oronasal interface into a peak flow meter (Access Peak Flow Meter, Cedar Grove, USA) with the tracheostomy tube covered and cuff deflated. If 160 l/min was not achieved then an assisted CPF was determined by removing the tube, covering the ostomy while ventilating the lungs by mouth piece NVS as needed, and having the patient air stack consecutive ventilator-delivered volumes via a mouth piece to a deep lung volume and then coughing into a peak flow meter as an abdominal thrust was applied (8). Patients unable to attain 120 l/m of CPF were not decanulated but were referred to otolaryngology for evaluation for reversible obstructive lesions. Patients with CPF between 120 and 160 l/m were advised against but not excluded from decanulation.
The time having used TMV and h/day of current use were recorded. End-tidal CO2 or PaCO2, if elevated, was normalized by increasing ventilator settings and reassigning physiologic back-up rates. Any supplemental oxygen was discontinued; the airways were suctioned, and the SpO2 and VC were measured (first VC data point). The VC was measured by setting the ventilator to CPAP-mode without pressure support and the maximal exhaled volume in multiple attempts recorded with the tracheostomy tube cuff fully inflated. The VCs of patients using ventilators without software for expiratory volume or having tracheostomy tubes without cuffs were measured by Wright Respirometer Mark 14 (Ferraris Medical, London, England) via oronasal interfaces with the tracheostomy tube out and the ostomy covered.
For those meeting criteria, whether at home or hospitalized, the tracheostomy tubes were switched to fenestrated ones that were capped. The patients were then placed on NVS via nasal interface for sleep and nasal or mouth piece NVS when awake. Assist/volume control mode was used with tidal volumes of 800 to 1,500 ml and normal physiological back-up rates for age. If the SpO2 baseline was less than 95%, MIE was initially used up to every 2 h at 50 to 70 cm H2O via the tube with the cuff, if present, inflated, until target ambient air SpO2 ≥ 95% was achieved. Medical and respiratory management regimens were unchanged during the peri-decanulation period. When the patients satisfied decanulation criteria and realized that they no longer needed tubes they were decanulated.
Decanulation criteria were satisfied by reversal of all acute illnesses, being medically stable and afebrile with a normal leucocyte count, having SpO2 ≥ 95% in ambient air and normal CO2 despite requiring up to continuous ventilatory support (6). Once achieved, VC and h/day of ventilator use were recorded (second VC data point) just before decanulation. Decanulations were performed in an outpatient physical medicine and rehabilitation clinic except for CTMV dependent patients who did not have overnight nursing services to monitor ambient air SpO2. They were decanulated in an intensive care unit.
Patients were decanulated to mouth piece or nasal CNVS as they preferred. Assist volume control mode at 800 to 1,500 ml volumes, rates of 10 to 12, and no end expiratory pressure were used. Occlusive pressure dressings were applied over the stoma to enable effective NVS and MIE without stomal leakage. Post-decanulation, home care providers administered MIE in the clinic or critical care unit at 35 to 60 cm H2O pressures upon patient demand up to every 30 min and as needed to maintain ambient air SpO2 ≥ 95% (4). The outpatients were monitored in the clinic for several hours to assure stable ambient air SpO2 ≥ 95% before returning home. The critical care patients were discharged directly home when medically stable with SpO2 ≥ 95% after ostomy closure and once their care providers were documented to be adept at NVS and MIE. All but 10 had already been using NVS and MIE for weeks to months at home prior to decanulation. Tracheostomy stomas were sutured if spontaneous closure did not occur. The part-time NVS users had SpO2 alarms set from 80 to 85% during sleep. Following discharge, patients who developed symptoms that suggested excessive air leakage during sleep underwent sleep end-tidal CO2 and SpO2 monitoring and were considered for conversion to NVS via oronasal interfaces.
After discharge the patients were re-evaluated in clinic within 3 weeks (third VC data point). Any decrease in h/day of need for NVS was documented. The patients had either weaned off of aid, weaned to less than 20 h/day of NVS, or remained CNVS dependent.
Successful decanulation was defined by never again requiring tracheotomy except for amyotrophic lateral sclerosis (ALS) patients with deteriorating bulbar-innervated musculature who would eventually require it to survive (14). Significance in mean differences in VC was determined by Student t-test. Weaning rates from CTMV to part-time NVS were also determined. Noting that some patients who had never used NVS became definitively CTMV dependent from tracheotomy but were able to wean following decanulation, we then reviewed the charts of 15 previously referred DMD TMV users who had not been offered decanulation.
Results
Sixty-seven TMV users presented for decanulation; 5 had maximum CPF less than 120 l/m and one with severe bronchiectasis was unable to achieve target SpO2 and were excluded. Six highly motivated patients with CPF between 120 and 160 l/m and all 55 with greater than 160 l/m were decanulated. The 61 patients to be decanulated included 34 who had been using tracheostomy ventilation for 18.1 ± 21.1 months and 27 who had been using it for 3 months or less. Thirty-six were CTMV dependent. Ten patients were decanulated as inpatients and 51 as outpatients.
On presentation 51 of the 61 patients had ambient air SpO2 baselines less than 95% even after airway suctioning and increasing ventilator settings to normalize CO2 levels. Ambient air SpO2 normalized for all only after MIE was introduced. This occurred at home in the 1 week to 3 months they practiced NVS and MIE before decanulation or in 1 to 3 days preceding decanulation for the 10 hospitalized patients. The patients had significant increases in VC from presentation, to meeting decanulation criteria, to the point of least diurnal NVS use within 3 weeks of decanulation (Table I). Table I further denotes the numbers of patients by diagnostic category who weaned from CTMV to part-time NVS following decanulation. The “CNVS dependence” column indicates the length of time during post-decanulation follow-up that the patients required CNVS whereas the “total NVS” column includes all post-decanulation NVS use categories including CNVS.
One DMD CTMV user was admitted for decanulation but initially failed to meet the SpO2 criteria and maximum CPF was less than 160 l/min. He continued to practice CNVS and MIE at home and was successfully decanulated to CNVS in the outpatient clinic 2 months later and has remained dependent on CNVS at home for 13 years. Only one SCI patient was re-canulated within 12 h despite having CPF over 160 l/m because of severe abdominal distension and cardiovascular instability associated with turns in bed and digital stimulation for bowel evacuations.
During a follow-up of a mean of 8 years per decanulated patient, none underwent re-tracheotomy. Eight patients died. Five ALS patients died 0.3, 6, 11, 11, and 16 months after discharge (mean 5.5 months (standard deviation; SD 4.1)) using CNVS and refusing re-tracheotomy despite deterioration of bulbar-innervated muscle function. One patient with DMD died from congestive heart failure after 16 months of CNVS. A spinal bifida CNVS user for 12 months and a nocturnal-only NVS user with polymyositis also died.
Eight of the 9 DMD patients in this study were CTMV dependent for a mean of 12.5 months (range 1–23) and the other used only nocturnal TMV prior to successful decanulation. Five of the 9 had become CTMV dependent from the point of tracheotomy despite no prior ventilator use. Decanulation to CNVS and MIE resulted in mean VC increasing by 47% (Table I) and permitted 8 to wean to part-time NVS. Of 15 previously referred DMD TMV users who underwent tracheotomy at a mean age of 21.5 years (SD 4.8) and were not offered decanulation, 8 became CTMV dependent immediately upon tracheotomy even though 6 of the 8 had not used any ventilatory assistance prior to tracheotomy and two had only been nocturnal bi-level positive airway pressure users before sudden CTMV dependence.
|
Table I. Peri-decanulation changes in vital capacity and breathing tolerance associated with mechanical insufflation-exsufflation (n = 61) |
|||||||||||||
|
Diagnosis |
Patients n |
Age at decanulation, years Mean (SD) |
Ventilator use (h/day) |
Vital capacity (ml) |
CPF ≥ 160 l/m before decanulation n |
Post extubation NVS (months) |
|||||||
|
At decanulation Mean (SD) |
CTMV n |
NVS minimum in 3 weeks post decanulation Mean (SD) |
Weaneda n |
Admission (data point 1) Mean (SD) |
At decanulation (data point 2) Mean (SD) |
Maximum in 3 weeks post decanulation (data point 3) Mean (SD) |
CNVS dependence Mean (SD) |
Total NVS use Mean (SD) |
|||||
|
ALS |
10 |
53.5 (13.9) |
15.6 (8.1) |
6 |
14 (8.3) |
4 |
625 (502) |
708 (409) |
865 (557) |
10 |
32.2 (47.8)c |
45.3 (44.9) |
|
|
DMD |
9 |
26.7 (7.2) |
22.4 (5.1) |
8 |
11.5 (5.8) |
6 |
180 (58) |
322 (168) |
474 (184) |
7 |
54.3 (32.4)d |
76.4 (44.1) |
|
|
non-DMD muscular dystrophies |
16 |
44.5 (12.6) |
17.9 (7.7) |
10 |
12.1 (6.5) |
9 |
444 (302) |
684 (394) |
791 (414) |
15 |
78.7 (69.6)e |
106.4 (69.2) |
|
|
oSMA |
3 |
39 (21.2) |
13.3 (7.3) |
0 |
8.0 |
0 |
613 (341) |
810 (340) |
988 (141) |
3 |
0 |
128 (35.4) |
|
|
oNMD |
8 |
54.4 (14.6) |
17.2 (7.6) |
4 |
8.0 |
4 |
585 (284) |
775 (411) |
1,162 (481) |
8 |
15.0 (9.9)f |
73.4 (65.0) |
|
|
Polio |
4 |
64.3 (7.1) |
12 (8) |
0 |
8.0 |
0 |
668 (459) |
990 (589) |
1,010 (412) |
4 |
52g |
115.5 (113.6) |
|
|
Obesity |
2 |
46 (2.8) |
16 (7.4) |
1 |
12.0 (5.7) |
1 |
1,525 (573) |
1,605 (361) |
2,195 (134) |
2 |
62g |
148.5 (38.9) |
|
|
RPS |
5 |
57.6 (16.3) |
21.4 (4.0) |
3 |
13.4 (6.1) |
2 |
418 (238) |
684 (248) |
1,002 (478) |
2 |
131g |
97.0 (39.4) |
|
|
SCIb |
4 |
43.4 (17.8) |
24 |
4 |
16 (6.6) |
0 |
332 (228) |
460 (259) |
775 (358) |
4 |
135 (157)f |
107.8 (95.8) |
|
|
Total |
61 |
47.7 (12.6) |
17.8 (7.3) |
36 |
11.4 (6.4) |
26 |
608 (308) |
865 (344) |
937 (383) |
55 |
70.0 (63.3) n = 32 |
100.2 (60.7) n = 60 |
|
|
aWeaned to less than 20 h/day of noninvasive intermittent positive pressure ventilatory support (NVS); bThere were 4 spinal cord injured patients decanulated but one was re-canulated due to severe abdominal distension and cardiovascular instability so the vital capacity and NVS data are for 3 SCI patients only. All patients were successfully decanulated except where noted. ALS: amyotrophic lateral sclerosis; DMD: Duchenne muscular dystrophy; non-DMD muscular dystrophies: myotonic, limb-girdle, facioscapulohumeral and Becker muscular dystrophies; oSMA-spinal muscular atrophy types 2, 3; oNMD: other neuromuscular disorders including myasthenia gravis, Guillain-Barré syndrome, non-muscular dystrophy myopathies; polio: post-poliomyelitis; obesity: obesity-hypoventilation syndrome; RPS: other restrictive pulmonary syndromes associated with multiple sclerosis, traumatic brain injury, critical care neuromyopathy; SCI: spinal cord injury; CPF: unassisted or assisted cough peak flows (see text); CNVS: continuous noninvasive ventilatory support. |
|||||||||||||
Discussion
”Noninvasive ventilation”, which is generally synomous with low spans of bi-level positive airway pressure, is inadequate for ventilatory support. Thus, ”noninvasive ventilation” is not used continuously, not considered ’support,’ nor is it considered an alternative to tracheostomy except in a handful of centers (4, 9–15). “Unweanable” patients with NMD or SCI, as well as many ventilator weaned patients, are often left with tracheostomy tubes (16–20) and spend the rest of their lives in skilled nursing facilities in the USA or with 16–24 h/day of home nursing at costs exceeding $300,000 per year (21, 22). Yet, CNVS and MIE have supported patients with DMD (4), SCI (10), post-polio survivors, and children with SMA type 1 (11) in their homes for 20 to 60 years in many cases and patients with ALS for up to 8 years (14). Many NVS users weaken to become CNVS dependent without ever being hospitalized and are, therefore, never evaluated for ongoing home nursing services. They remain home, as they prefer it, with or without paid attendant services, at greatly reduced expense, and with preserved quality of life.
Conversion from invasive to noninvasive ventilation requires maintaining normal alveolar ventilation and SpO2 breathing room air. Since any desaturation in ambient air indicates some combination of hypoventilation, airway secretion encumbrance, and residual pulmonary disease, maintaining SpO2 ≥ 95% is a high priority criterion for decanulation or extubation of these patients (5). In addition, airway suctioning cannot substitute for the cough flows needed to clear the 6 central airway divisions (13). Therefore, liberation from invasive ventilation requires appropriate patient selection and use of mechanical aids for coughing and breathing, namely MIE and NVS. We consider that our decanulation successes were principally due to normalizing ambient air SpO2 by using MIE via the tubes and then decanulating to full CNVS and continuing MIE to keep SpO2 normal. In addition, two of the authors have 30 years of experience in noninvasive respiratory management. Although relying on nasal NVS for sleep results in frequent brief arousals of which patients are unaware (23), they are not more likely to occur after decanulation than they are to occur before it so they were not an immediate concern.
It had been felt that inability to achieve assisted CPF greater than 160 l/m signaled insufficient airway patency, most often due to severe bulbar-innervated muscle dysfunction, to permit effective use of MIE post-extubation or decanulation (8). However, in this study 6 of the 60 patients who were successfully decanulated had CPF between 120 and 160 l/m whereas one patient with CPF greater than 160 l/m failed, thus 160 l/m cannot be considered an absolute marker for success or failure. Indeed, in a recent study, 155 of 157 “unweanable” patients with neuromuscular weakness were successfully extubated with only one who had assisted CPF > 160 l/m failing because of cardiovascular instability and many with unmeasurable CPF, including infants with spinal muscular atrophy type 1, succeeding (5). With great experience in noninvasive management it is possible to extubate and decanulate patients with CPF < 160 l/min, however, the less the upper airway patency as suggested by low CPF or MIE flows, the less effective is MIE in keeping the airways clear after extubation or decanulation and the greater the risk of failing noninvasive management. Although it is possible to extubate or decanulate patients with severe bulbar ALS and no measurable CPF (5), we do not recommend it for centers with little or no experience in noninvasive management.
Effective MIE pressures via the upper airways have been reported to be 40 mm Hg (54 cm H2O) (6, 24–27). Use of MIE has not been previously described via tracheostomy tubes. Although many clinicians are concerned when using these insufflating pressures, no clinically apparent barotrauma was observed during this or our previous studies (1, 2, 4, 5, 8). Its safety was corroborated when we reviewed 1,000 outpatients with NMDs who air stacked three times daily to up to 80 cm H2O pressures and in some cases for over 50 years with no apparent adverse effects (28).
Reports as early as 1955 indicated that patients became permanently ventilator dependent after tracheotomy. Patients using iron lungs for sleep who had VCs of 500 to 600 ml could breathe unaided during the day but became CTMV dependent after tracheotomy (9). Airway secretions caused by the tracheostomy tubes decrease gas exchange and VC and impose the need for routine airway suctioning an average of 8 times a day (5). The facts that CTMV leads to hyperventilation possibly by bypassing upper airway afferents (29) and inspiratory muscle deconditioning (31) also contribute to increased ventilator dependence. Indeed, 13 of 25 consecutively referred DMD patients became CTMV dependent immediately after tracheotomy despite little or no ventilator use before it (8). Conversely, 26 of 36 patients who were CTMV dependent for months before decanulation weaned to part-time NVS in 3 weeks or less. The fact that 51 patients were successfully decanulated in an outpatient clinic places this into the realm of the physiatrist or any physician who wishes to master these techniques and manage patients long-term rather than the intensivist for most cases.
Although lack of blinding and controls are limitations of this study, controls and randomization to noninvasive support are not ethically possible when decanulating patients with no ability to breathe. Previous management using bronchodilators, airway suctioning, chest physical therapy, and ventilator weaning protocols did not result in successful weaning, extubation, or decanulation but transition to CNVS and MIE did. For almost every case in which the ambient air SpO2 baseline was less than 95% upon presentation despite conventional management, aggressive MIE resulted in the immediate expulsion of copious mucopurulent secretions and often increases in VC and SpO2. Thus, the combination of MIE and CNVS permits a paradigm shift from invasive tubes to noninvasive long-term management for patients who satisfy specific criteria, and from a paradigm of weaning to permit decanulation to decanulation to permit self-weaning.
References
