Tanja Karic, MD1,2, Cecilie Roe, MD, PhD1,4, Tonje Haug Nordenmark, PhD1, Frank Becker, MD, PhD3,4 and Angelika Sorteberg, MD, PhD2.4
From the Departments of 1Physical Medicine and Rehabilitation and 2Neurosurgery, Oslo University Hospital, Oslo, 3Sunnaas Rehabilitation Hospital, Nesoddtangen and 4Institute of Clinical Medicine, University of Oslo, Oslo, Norway
OBJECTIVE: To assess the impact of early mobilization and rehabilitation on global functional outcome one year after aneurysmal subarachnoid haemorrhage.
METHODS: Prospective, controlled, interventional study comprising patients managed in the neuro-intermediate ward following repair of a ruptured intracranial aneurysm. Patients in the Control group (n = 76) received standard treatment, whereas those in the Early Rehab group (n = 92) in addition underwent early mobilization and rehabilitation. Demographic, clinical and intervention data were registered. Global functional outcome was assessed using the modified Rankin Scale and the Glasgow Outcome Scale Extended.
RESULTS: The 2 groups were similar in their demographic and clinical characteristics. Early Rehab group patients were mobilized more quickly (p < 0.001), median 1.4 days (range 0–23 days) after aneurysm repair. After 1 year, 47% of the patients had made a good recovery, whereas 6.5% had died. Regression analysis did not reveal any significant effect of early rehabilitation on functional outcome. However, in poor-grade patients, early rehabilitation more than doubled the chance of a favourable outcome (adjusted odds ratio = 2.33; confidence interval 1.04–5.2, p = 0.039).
CONCLUSION: Early mobilization and rehabilitation probably increases the chance of a good functional outcome in poor-grade aneurysmal subarachnoid haemorrhage patients.
Key words: early rehabilitation; mobilization; functional outcome; subarachnoid haemorrhage.
J Rehabil Med 2016; 48: 00–00
Correspondence address: Tanja Karic, Department of Physical Medicine and Rehabilitation, Oslo University Hospital, Ullevaal and Rikshospitalet, P.B. 4950 Nydalen, NO-0424 Oslo, Norway. E-mail: tanja.karic@gmail.com
Accepted May 17, 2016; Epub ahead of print Aug 5, 2016
INTRODUCTION
Aneurysmal subarachnoid haemorrhage (aSAH) is a dramatic event globally affecting the brain. Depending on the severity of the haemorrhage, aSAH is associated with up to 50% mortality (1, 2). Despite improvements in surgical and endovascular aneurysm repair, intensive care and rehabilitation, the resulting long-term disability is still significant, with up to 40% of patients being unable to return to their premorbid occupation (3, 4). Predicting and, more importantly, improving long-term functional outcome is therefore of major importance.
According to recent research, early-introduced rehabilitation improves functional outcome in patients after stroke and traumatic brain injury (5–12). In the context of aSAH, however, early rehabilitation has not yet been incorporated into treatment guidelines (13, 14). Traditionally, bed rest has been prescribed after aSAH due to the fear that head elevation in excess of 45° could decrease cerebral perfusion and give rise to delayed cerebral ischaemia. Mobilization was feared to potentially cause re-bleeding and negatively affect the development and course of cerebral vasospasm; this still represents the most feared complication of aSAH (15). On the other hand, Riordan et al. (16) concluded in laboratory and clinical models that mild exercise reduces cerebral vasospasm. Early rehabilitation after aSAH was deemed both safe and feasible in the studies of Olkowski et al. (17) and Karic et al. (18). A mobility intervention also improved functional outcome at discharge in a mixed patient population with intracerebral haemorrhage and aSAH (19). The long-term effect of early-introduced rehabilitation in aSAH patients, however, needs to be further investigated.
Hence, the aim of the present study was to assess the impact of early rehabilitation on global functional outcome one year after aSAH.
MATERIAL AND METHODS
Study design
This prospective, controlled, interventional study included patients managed in the neuro-intermediate ward (NIW) of the Neurosurgical Department, Rikshospitalet, Oslo University Hospital, Oslo, Norway, following repair of a ruptured intracranial aneurysm between January 2011 and December 2012. NIW is an intensive care unit that cares for vascular neurosurgical patients only, requiring no more than non-invasive respiratory support. When there was no further need for cerebrospinal fluid drainage and the risk of development of cerebral vasospasms was considered low, patients were discharged from the NIW to a local hospital and from there to a rehabilitation institution, nursing home or their home. Patients still in need of invasive respiratory support were cared for in the general intensive care unit. Patients who needed prolonged intensive respiratory support, but no further neurosurgical care, were directly transferred to the ICU at their respective local hospital and were not included in this study. Patients admitted during 2011 received standard treatment in accordance with our institutional guidelines (20). They constitute the Control group. Patients admitted during 2012 underwent early rehabilitation in addition to standard treatment. They comprise the intervention group, hereafter referred to as the Early Rehab Group.
The present study was approved by the Regional Committee for Medical Research Ethics, South-East Norway, archive number 2011/2189, Clinical Trials number 0925-0586 (ClinicalTrials.gov identifier NCT01656317). Oral and written consent was obtained from all patients included in the study.
Patients
Inclusion criteria: adults (> 18 years) from the South-East health region with aSAH who were admitted to the NIW at Oslo University Hospital after aneurysm repair.
Exclusion criteria: previous SAH, traumatic brain injury or neurodegenerative disease that could interfere with aSAH-acquired disability.
INTERVENTION: Mobilization and early rehabilitation
Early rehabilitation was based on an inter-disciplinary approach (21) and initiated upon arrival to the NIW. The content of the early rehabilitation programme has been described earlier (18) and, amongst others, included: mobilization, prevention of contractures by passive exercises and adequate postural changes during rest and sleep, guidance in activities of daily living and swallowing and eating, pulmonary rehabilitation, body exercises and balance training, reality orientation and information and emotional support to patients and their families. Patients were also shielded from overstimulation and care was taken to provide breaks between activities (18).
The core component of the early rehabilitation intervention was the gradual increase in mobilization level according to an algorithm developed for the early phase after aSAH, and hence taking into account the presence and degree of cerebral vasospasm (18). According to the literature, cerebral vasospasm occurs from the third day after the ictus and may peak between days 7 and 10 after aSAH (22). With regard to cerebral vasospasm, all our patients are screened with cerebral computed tomography angiography on day 7 after the ictus. Therefore, we chose the level of mobilization achieved on days 4 and 7 when comparing mobilization levels between groups.
All patients were routinely invited to follow-up by the neurosurgeon and the interdisciplinary team approximately 3 and 12 months after aSAH; here they received recommendations about further rehabilitation and return to work.
Assessments
Demographic data (age, sex, education, employment) were obtained from inpatient medical records, questionnaires and interviews with patient/family.
Clinical status was scored in terms of a Hunt and Hess grade (HH) (23) just prior to aneurysm repair and in terms of the World Federation of Neurosurgery Scale (WFNS) (24) upon arrival at NIW after aneurysm repair. Patients were considered to be in clinical good-grade when in WFNS grades 1 or 2 and correspondingly being poor-grade when in WFNS grades 3–5.
Clinical and radiological features, as well as progress in mobilization and length of stay, were obtained from inpatient medical records. The fraction of patients who achieved mobilization to the edge of the bed and to a chair, respectively, on days 4 and 7 after aneurysm repair was used to compare differences in mobilization between groups.
Outcome measurement
Global functioning as a main outcome was evaluated using the Modified Rankin Scale (mRS) (25) and the Glasgow Outcome Scale Extended (GOSE) (26).
Both mRS and GOSE were assessed one year after aSAH in a structured interview with patients and/or nursing staff /family. For patients who did not complete 12 months of follow-up, the mRS/GOSE scores were based on medical records and those scores were included in the analyses. For analysis purposes, mRS and GOSE scores were categorized into 4 categories. Table I shows the consistency and discrepancy in patient function measurements between the 2 scales.
|
Table I. Categories of Modified Rankin Scale (mRS) and Glasgow Outcome Scale Extended (GOSE) |
||||
|
mRS score |
Level of function |
Category |
GOSE score |
Level of function |
|
0 |
No limitations and no symptoms |
4 Good recovery |
8 |
Upper good recovery; No limitations and no symptoms. |
|
1 |
No significant disability: Minor symptoms, able to carry out normal activities. |
7 |
Lower good recovery; Minor symptoms, able to carry out normal activities. |
|
|
2 |
Slight disability: Returning to work with reduced capacity or not able to work. Reduced or unable to participate in leisure and social activities. |
3 Moderate disability |
6 |
Upper moderate disability; Returning to work with reduced capacity, reduced participation in leisure and social activities. |
|
3 |
Moderate disability: Need assistance to look after own affairs (preparing simple meal, basic household chores, looking after household expenses, shopping and travelling locally). |
5 |
Lower moderate disability; Able to work only in sheltered workshop or non-competitive job. Unable to participate in leisure and social activities. |
|
|
4 |
Moderate severe disability: Need assistance to attend bodily needs/ for walking. |
2 Severe disability
|
4 |
Upper severe disability; Some dependence on others but could care for themselves for up to 8 h. Need assistance for travelling and shopping locally. |
|
5 |
Severe disability: Constant care; bedridden, may be incontinent and requiring constant care. |
3 |
Lower severe disability; Complete dependence of others. Cannot care for themselves for up to 8 h if necessary but do not need constant care. |
|
|
2 |
Vegetative state. |
|||
|
6 |
Dead |
1 Dead |
1 |
Dead |
Statistical analysis
Descriptive data were presented using proportions and mean values with standard deviations or median with range. Mann-Whitney U test and χ2 test were used to compare differences between the Early Rehab group and the Control group in terms of sex, age, aSAH characteristics, length of hospital stay and functional outcome at 1-year follow-up. The same comparisons were conducted for patients who completed the programme vs drop-outs at the 12-month follow-up.
The adjacent category logistic regression model fit best to our data and was therefore used to evaluate the effect of early rehabilitation on functional outcomes with mRS and GOSE one year after aSAH as the dependent variables. Independent variables were: age, sex, severity of aSAH (assessed by Hunt and Hess prior to aneurysm repair), mode of treatment (surgical vs endovascular) and time to 1-year follow up (in days). The models were checked for multicollinearity and run for all patients, as well as separately for good-grade and poor-grade patients. A 2-sided significance level of 5% was used.
Statistical analyses were performed using SPSS for Windows, version 22 (SPSS Inc., Chicago, IL, USA) and for regression analysis: Stata 13 (Stata Corp LP, College Station, TX, USA).
Sample size calculations were based on the results of early rehabilitation provided to patients with severe traumatic brain injury (TBI) in our hospital (5), with a 50% higher probability for favourable outcome in the Early Rehab group according to the GOSE score. Given 90% power and a significance level of 0.05, 25 patients in each group would be necessary. Between120 and 140 patients with aSAH are admitted to Oslo University Hospital each year. We assumed that 70% of the patients would meet the inclusion criteria and that 10% would drop out at 1 year. Also, considering seasonal variations in aSAH incidence (27) and our consecutive cohort design, a data collection period of one year for each group would ensure a sufficient number of study participants.
RESULTS
Patients and clinical features
A total of 81 patients from 2011 (Control group) and 98 patients from 2012 (Early Rehab group) were eligible for inclusion. Four patients from the Control group and 5 from the Early Rehab group were excluded due to previous SAH. One patient in the Control group refused to participate in the study and 1 patient from the Early Rehab group was excluded due to degenerative central nervous system (CNS) disease. Hence, 76 patients were included in the Control group and 92 in the Early Rehab group. Five Control group patients and 4 Early Rehab group patients did not attend the 12-month follow-up, but were included in the study. Non-attenders had a mean age of 57 years, 67% were women, the median Hunt and Hess grade was 3, and the length of stay (LOS) at our hospital was median 11 days (range 5–27 days). Non-attenders did not differ substantially from patients attending the 12-month follow-up in terms of demographics, clinical features and LOS at our hospital (p > 0.05); none of the non-attenders were employed before aSAH, which was significantly different (p < 0.001) from the rest of the patients (50% in Control group and 63% in Early Rehab group were employed before aSAH).
The demographics and aSAH characteristics in the Control and Early Rehab groups are presented in Table II. There were no statistically significant differences between the groups. LOS at our hospital was also similar, with a median of 14.5 days (range 2–61 days) in the Control group and 14.4 days (3–37 days) in the Early Rehab group. However, median time to 1-year follow-up after aSAH was 413 days (range 320–564 days) in the Control group and 384 days (range 346–507 days) in the Early Rehab group (p = 0.000).
|
Table II. Demographic and clinical characteristics |
||
|
Variables |
Control group (n = 76) |
Early rehabilitation group (n = 92) |
|
Demographic characteristics |
||
|
Age, years, mean (range) |
54 (25–79) |
56 (25–81) |
|
Sex, male/female, % |
37/ 63 |
30/ 70 |
|
Years of education, % ≤ 12 years > 12 years |
80 20 |
68 32 |
|
Clinical characteristics |
||
|
Aneurysmal source of bleeding, % Anterior cerebral arteries Middle cerebral – and internal carotid arteries Vertebro-basilar arteries |
50
37 13 |
46
40 14 |
|
Surgical aneurysm repair, % |
50 |
47 |
|
Hunt and Hess score just prior to aneurysm repair, % of patients 1 2 3 4 5 |
28 36 14 17 5 |
22 37 23 11 7 |
|
WFNS at transfer to NIW, % of patients 1 2 3 4 5 |
33 29 22 13 3 |
33 34 16 14 3 |
|
WFNS: World Federation of Neurological Surgeons Score; NIW: neuro-intermediate ward. |
||
Mobilization and early rehabilitation
The mobilization level upon transfer to NIW was at median zero (bed rest with head elevation 30°) in both poor- and good-grade patients. In poor-grade patients, (WFNS3, 4 and 5) early rehabilitation was initiated after a median of 7.4 days (range 1–23 days) and in good-grade patients after 0.9 days (range 0–20 days). Early rehabilitation was applied for a median of 9 days (range 1–36 days) in good-grade patients and a median of 10 days (1–26 days) in poor-grade patients.
Progression of mobilization was more rapid in the Early Rehab compared with the Control group. Significantly more patients in the Early Rehab group were mobilized to the edge of bed or to a chair on day 4 and day 7 after aneurysm repair (Fig. 1).
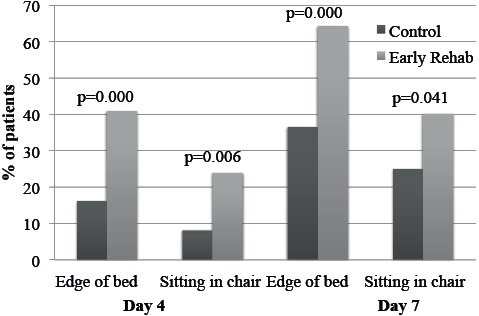
Fig. 1. Proportion of patients mobilized to sitting at the edge of bed/sitting in a chair on days 4 and 7 after aneurysm repair.
Functional outcome and effect of mobilization and early rehabilitation
Functional outcome for all patients combined for mRS and (GOSE) was as follows: good recovery 47% (47%), moderate disability 38% (36%), severe disability 8% (10%), and mortality 6.5%.
Neither the unadjusted nor adjusted model showed an effect of early rehabilitation. Increasing age reduced the probability of better outcome. Good clinical status prior to aneurysm repair (Hunt and Hess) increased the probability of better outcome (adjusted odds ratio (OR) 1.39) (Table III).
|
Table III. Effect of early rehabilitation on functional outcome as measured by modified Rankin Scale at one year follow-up in all patients (n = 168) |
|||||
|
Good recovery 79 Moderate disability 64 Severe disability 14 Dead 11 |
Adjacent-category logistic regression for all patients |
||||
|
Univariate |
|
Multivariate |
|||
|
Unadjusted OR (95% CI) |
p-value |
|
Adjusted OR (95% CI) |
p-value |
|
|
Early rehabilitation |
0.982 (0.69–1.39) |
0.922 |
|
1.30 (0.836–2.037) |
0.242 |
|
Age (years) |
0.95 (0.93–0.97) |
0.000 |
|
0.95 (0.929–0.972) |
0.000 |
|
Hunt and Hess grade (23) |
1.38 (1.18–1.63) |
0.000 |
|
1.39 (1.16–1.67) |
0.000 |
|
Time to 12 months follow-up (days) |
1.00 (0.99–1.01) |
0.102 |
|
1.00 (0.999–1.01) |
0.052 |
|
Surgical aneurysm repair |
1.01 (0.711–1.43) |
0.962 |
|
1.25 (0.823–1.904) |
0.292 |
|
Male sex |
0.98 (0.67–1.42) |
0.900 |
|
1.189 (0.756–1.872) |
0.453 |
|
Significant values are shown in bold. |
|||||
The same results were also found in adjacent-category logistic regression analysis with categorized GOSE as the dependent variable (results not presented).
When dichotomizing patients according to clinical status at arrival NIW into poor-grade (WFNS 3,4,5) and good-grade (WFNS 1,2), a statistically significant effect of early rehabilitation was found among poor-grade patients, with adjusted OR = 2.33 (95% CI 1.04–5.2, p = 0.039) for favourable outcome (Table IV). Hunt and Hess grade prior to aneurysm repair as an independent variable was excluded in this model due to high correlation with WFNS used for dichotomizing. Among the patients who were in a good clinical grade at transfer to NIW after aneurysm repair, age was the only statistically significant predictor of functional outcome in the multivariate analysis (Table IV).
|
Table IV. Effect of early rehabilitation in good-grade vs poor-grade patients on functional outcome as measured by categorized modified Rankin Scale at 1-year follow-up |
|||||
|
Characteristics |
Adjacent-category logistic regression |
||||
|
Univariate |
|
Multivariate |
|||
|
Unadjusted OR (95% CI) |
p-value |
|
Adjusted OR (95% CI) |
p-value |
|
|
Patients in poor clinical status (WFNS 3,4,5) after aneurysm repair at arrival neuro-intermediate ward (n = 60) Good recovery = 19; Moderate disability = 23; Severe disability = 11; Dead = 7 |
|||||
|
Early rehabilitation performed |
1.007 (0.60–1.689) |
0.979 |
|
2.33 (1.04–5.2) |
0.039 |
|
Age (years) |
0.942 (0.911–0.974) |
0.000 |
|
0.93 (0.89–0.96) |
0.000 |
|
Time to 12-month follow-up (days) |
1.000 (–0.002–0.018) |
0.137 |
|
1.00 (0.99–1.02) |
0.064 |
|
Surgical aneurysm repair |
0.964 (0.570–1.632) |
0.894 |
|
1.07 (0.55–2.08) |
0.843 |
|
Male sex |
0.929 (0.530–1.628) |
0.799 |
|
0.93 (0.44–1.97) |
0.848 |
|
Patients in good clinical status (WFNS 1, 2) after aneurysm repair at arrival neuro-intermediate ward (n = 108) Good recovery=60; Moderate disability = 41; Severe disability = 3; Dead = 4 |
|||||
|
Early rehabilitation performed |
0.885 (0.52–1.507) |
0.65 |
|
1.02 (0.57–1.85) |
0.934 |
|
Age (years) |
0.968 (0.942–0.995) |
0.023 |
|
0.96 (0.93–0.99) |
0.012 |
|
Time to 12 months follow-up |
1.00 (–0.006–0.011) |
0.558 |
|
1.00 (0.997–1.010) |
0.265 |
|
Surgical aneurysm repair |
1.365 (0.782–2.382) |
0.273 |
|
1.56 (0.86–2.83) |
0.145 |
|
Male sex |
1.06 (0.617–1.829) |
0.826 |
|
1.30 (0.71–2.36) |
0.395 |
|
Significant values are shown in bold. WFNS: World Federation of Neurosurgery Scale (27). |
|||||
DISCUSSION
The core finding of the present study was that early rehabilitation was not harmful. We found no significant impact of early rehabilitation on global functional outcome; however, in the subgroup of poor-grade patients early rehabilitation increased the chance of a better global functional outcome one year after the ictus. Hunt and Hess grade just prior to aneurysm repair and age were additional predictors of outcome.
Patients, aSAH characteristics and clinical features
Our patients did not differ from typical aSAH patients as described in the literature (16) (28–31), i.e. being middle-aged with female predominance, with less than one-third having higher education and less than 60% being employed before the ictus. Furthermore, the majority of patients were in a good clinical grade and their haemorrhage was likely to be caused by an aneurysm on the anterior cerebral arteries.
The Control group and the Early Rehab group were similar despite the lack of randomization. This was as expected because we collected data from all patients admitted throughout one year, and one could assume that aSAH populations from 2 consecutive years would be very similar.
Mobilization and early rehabilitation
The higher mobilization level achieved in the Early Rehab group confirms that we managed to implement our standardized early rehabilitation protocol into neurointensive care after aSAH.
The content of early rehabilitation in the poor-grade patients was similar to that described for patients after severe TBI (5). Analysing the content of early rehabilitation in aSAH patients showed that it was dependent on patients’ clinical status, in particular on the presence of a motor deficit and the content of early rehabilitation was different in poor- and good-grade patients (18). Postural changes during rest and sleep, exercises for contracture prevention, guiding in activities of daily living and assessment of swallowing and eating were almost exclusively used in patients with motor deficits (18). Therefore, when studying the impact of early rehabilitation, the dichotomization into good- and poor-grade patients should take into account the presence of a significant motor deficit. WFNS grade 3 requires the presence of a significant motor deficit and we therefore defined WFNS grades 3, 4 and 5 as poor-grade. From a rehabilitation perspective it was also important to consider the combined effect of the haemorrhage plus possible damage from the aneurysm repair. For instance, it would be very relevant for the content of rehabilitation if a patient admitted in Hunt and Hess grade 2 developed cerebral infarction and hemiparesis in conjunction with aneurysm repair. Despite being a good-grade patient at arrival, this patient would need rehabilitation efforts as a poor-grade patient due to the significant deficit that arose as a result of the aneurysm repair. To safeguard this perspective, we chose clinical grade after aneurysm repair at arrival at the NIW when our intervention was initiated), rather than the Hunt and Hess grade just prior to aneurysm repair.
Global functional level 1 year after aSAH
The modified Rankin Scale (mRS) (25) is a well-established tool for evaluating outcomes after stroke and aSAH, whereas the Glasgow Outcome Scale Extended (GOSE) (26) is extensively used in acquired brain injury-related disability. We chose to use both the mRS and GOSE in the assessment of global outcome one year after aSAH to display the different facets of the 2 assessment tools. The categorization of mRS and GOSE was rather similar, supporting comparison of results across these scales at the category level.
Global functional outcome for all patients (from both groups) in the present study corroborates earlier reports that most patients who survive the first weeks after aSAH are functionally independent (mRS < 4) one year after the ictus (2, 28, 32, 33).
Seventy percent of the patients who were in poor clinical grade before initiation of early rehabilitation regained independence in activities of daily life (mRS < 4). More than half (55%) of the patients in good clinical grades achieved a good recovery and 94% regained independence in activities of daily life (mRS < 4). Hence, in good-grade patients, global outcome measures, such as mRS and GOSE, probably have a ceiling effect.
Effect of mobilization and early rehabilitation on functional level 1 year after aSAH
Patients in poor-grade before initiation of early rehabilitation were more than twice as likely to achieve a favourable outcome if they had undergone early rehabilitation. A probable explanation is that poor-grade patients needed motor recovery, which early rehabilitation managed to improve by stimulating neuronal plasticity. Our findings corroborate with the study of Andelic et al. (5), suggesting that early rehabilitation implemented from a median of 12 days after the trauma improves outcome in patients with severe TBI. Likewise, the potential to induce brain plasticity is greater in the early stages after brain injury (34). On the other hand, a certain degree of spontaneous recovery in the sub-acute phase following TBI has been described (35). Spontaneous recovery may also have had an impact on functional outcome one year after aSAH, although we were unable to find reports on that topic.
Early rehabilitation did not improve functional outcome in patients who were in good-grade before initiation of early rehabilitation. A possible reason to that is the patients’ clinical state at admission being the strongest predictor of outcome (36) and any additional beneficiary intervention would not be able to improve the outcome at global outcome scales such as mRS and GOSE due to ceiling effect. Such changes could possibly have been detected with neuropsychological tests or quality of life questionnaires (3). On the other hand, our findings also indicate that early mobilization and rehabilitation did not have a negative impact on long-term outcome. A high proportion of these patients were already mobilized into a chair 4 days after aneurysm repair; i.e. in a phase where delayed ischaemic complications can occur (22). A higher incidence of such negative events would most likely have led to impairments in long-term global functioning.
Our statistical model was adjusted for variables that could interfere with outcome, including age, Hunt and Hess grade at admission, type of aneurysm repair, and sex. This choice of possible confounders in the multifactorial analysis was based on the review article by Jaja et al. (37). Presently, increasing age and poor clinical status assessed by the Hunt and Hess score at admission negatively influenced functional outcome, which is consistent with other studies (38). We also included the time to 1-year follow-up as a confounder because it could interfere with the aspect of spontaneous improvement. In fact, patients in the Early Rehab group had their 1-year assessment earlier than those in the Control group. This could have led to an underestimation of the impact of early rehabilitation if time is a factor for spontaneous improvement. Nevertheless, time to assessment failed to be a significant predictor of outcome in the multifactorial analysis.
Study limitations and strengths
There are several limitations to interpreting the results of the present study. We could not use randomization of patients because it was impossible to blind the caregivers to group allocation. There would also have been a considerable contamination effect by introducing early rehabilitation in a subgroup of patients within the same department. Inclusion of another neurosurgical department would have introduced new confounders regarding differences in treatment. Our groups received very standardized treatment with the only difference of early rehabilitation not being applied in the Control group. There were no obvious changes in treatment, hospital environment, or the availability of late rehabilitation between the years 2011 and 2012. Hence, we believe our study design is the best possible given the circumstances. With our inclusion of patients at arrival NIW, the present study missed the patients who needed prolonged care in the ICU and who were transferred directly to the ICU at a local hospital. This also means that some of the patients in the poorest clinical condition were not studied, which is reflected in the relatively low percentage of patients with Hunt and Hess grade 5. Inclusion of all of these patients could possibly have provided even clearer statistical results with regard to the impact of early rehabilitation. When introducing the concept of early rehabilitation in aSAH patients, we started at the NIW to provide the best possible control with regard to possible harmful effects and because most patients are treated here. Also, the treatment is more standardized than at the ICU (which was important when comparing 2 consecutive cohorts). Further studies should include patients who need prolonged intensive care and focus on the effectiveness and timing of specific rehabilitation efforts.
The traditional approach to analysis of functional outcome after TBI and stroke is to categorize the mRS and GOSE scores at a fixed point into categories. This approach causes loss of information and reduces the sensitivity of analyses. For a patient with a very severe injury, for example, survival may be a relevant endpoint, whereas for patients with less severe injuries any outcome worse than good recovery might be considered unfavourable. However, categorizing was a rational way of organizing the data for statistical purposes and also allowed a more comprehensive approach to using both mRS and GOSE as outcome measures. At the same time we are aware that global outcome measures like mRS and GOSE are not sensitive enough to capture cognitive outcomes that may have been influenced by early rehabilitation. Because of that, the results from this study should be interpreted with caution. Nevertheless, we further examined the ceiling effect in the poor-grade group with clinically poor prognostic potential and found that patients in poor-grade indeed had a better functional outcome in terms of mRS when they received early rehabilitation.
Conclusion
The present study revealed no harm or significant effect of early rehabilitation on global functional outcome one year after the ictus. In the subgroup of poor-grade aSAH patients, however, early rehabilitation increased the probability of a better global functional outcome. Further studies should focus on the effectiveness and timing of specific rehabilitation efforts and include patients who need prolonged intensive care.
REFERENCES
