Hsiao-Han Wang1, Che-Wei Liu2, Yu-Chuan Li1 and Yu-Chen Huang1
1Department of Dermatology, Wan Fang Hospital, Taipei Medical University, and 2Department of Surgery, Cathay General Hospital, Taipei, Taiwan
This meta-analysis examined the efficacy of different dosing regimens containing rituximab (RTX) in treating pemphigus. The analysis included 578 patients with pemphigus from 30 studies. Seventy-six percent of patients achieved complete remission after one cycle of RTX. Mean time to remission was 5.8 months, with a remission duration of 14.5 months and a 40% relapse rate. Eighteen patients (3.3%) developed serious adverse effects. The pooled estimate showed no significant differences in complete remission and relapse rates between patients treated with high-dose (near or ≥ 2,000 mg/cycle) vs. low-dose (< 1,500 mg/cycle) RTX. In the fully adjusted analysis, high-dose RTX was associated with longer duration of complete remission compared with low-dose RTX. No superiority of lymphoma protocol over rheumatoid arthritis or high-dose RTX over low-dose RTX was shown in other outcomes. RTX treatment is efficacious and well-tolerated in treating pemphigus. High-dose RTX treatment may lead to longer duration of remission. However, the choice of optimal regimen depends on the overall condition of the individual patient. Key words: pemphigus; rituximab; lymphoma protocol; rheumatoid arthritis protocol; immunoadsorption.
Accepted Apr 15, 2015; Epub ahead of print Apr 17, 2015
Acta Derm Venereol 2015; XX: XX–XX.
Yu-Chen Huang, Department of Dermatology, Wan Fang Hospital, Taipei Medical University, NO.111, Section 3, Hsing-Long Rd, Taipei 116, Taiwan, ROC. E-mail: dhist2002@yahoo.com.tw

*Partial content of this article was presented at World Congress of Dermatology 2015.
Pemphigus is a potentially fatal autoimmune mucocutaneous blistering disease. The therapeutic mainstay in pemphigus is systemic glucocorticoids in conjunction with other immunosuppressive agents (ISA), intravenous immunoglobulin (IVIG) and immunoadsorption (IA) (1, 2). However, some patients develop resistance to conventional therapy or experience steroid-related side-effects.
Rituximab (RTX), a chimeric anti-CD20 monoclonal antibody that induces B-cell depletion, has been used in the treatment of pemphigus with promising response (3). However, there is no universally accepted protocol for its use in pemphigus. Several commonly used regimens include a lymphoma protocol (4 weekly infusions of RTX at a dose of 375 mg/m2) (4–17), a rheumatoid arthritis (RA) protocol (2×1,000 mg doses of RTX at a 2-week interval) (10, 12, 18–21), low-dose RTX (2×500 mg doses of RTX at a 2-week interval) (10, 16, 21–24) with or without concomitant use of IA (9, 10, 25–29) or IVIG (30). RTX has also been used as a first-line treatment with favourable outcomes (12, 31). The complete and clinical remission rates in the previous reviews were approximately 65–78% (3, 32, 33) and relapse rates varied from 12% to 60% (3, 20, 32, 33). Serious complications related to RTX therapy were reported in only a small number of patients (3, 32, 33). Few studies have compared different treatment regimens. Although there are some indications that clinical outcomes may be improved with higher doses of RTX (16, 21, 22), uncertainty remains regarding the optimal RTX regimen. The aim of this research was to perform a systemic review and meta-analysis to investigate the clinical efficacy and adverse effects, and to compare the clinical outcomes of different RTX treatment regimens.
Materials and Methods
Data sources and search strategy
We searched PubMed, MEDLINE, EMBASE, and the Cochrane Library (Cochrane Database of Systematic Reviews, Database of abstracts of Reviews of Effects, Cochrane Controlled Trials Register, and Health Technology Assessment Databases) from inception to 31 October 2014, using the search terms “pemphigus foliaceus”, “pemphigus vulgaris”, or “pemphigus” combined with “rituximab”. Only human clinical studies written in English were included in the analysis.
Selection and outcomes
The literature search focused on randomized controlled trials comparative studies, and case series in which 5 or more patients with pemphigus (pemphigus foliaceus, PF; pemphigus vulgaris, PV) received RTX treatment. Review articles, case reports, guidelines, consensus manuscripts, correspondence, and letters to the editor were excluded. Subjects under 18 years old and using extremely high doses of RTX (≥ 6 weekly infusions of 375 mg/m2 RTX in a single cycle) were excluded. The outcomes assessed were complete remission (CR) rate after the first cycle of RTX, time to disease control (TDC), time to CR on or off therapy (TCRon or TCRoff), duration of CR, and relapse rate.
Data extraction
Data were independently extracted by 2 authors (HHW and YCH). Any disagreement was resolved by consensus. For studies that included multiple types of autoimmune bullous disease, only information about patients with PF and PV were extracted. Data on the following measures were extracted: study design, sample size, trial duration, treatment regimen, and concomitant therapy (Table SI1). Patients were further grouped into high-dose and low-dose groups according to the total dose of one cycle of RTX therapy. The high-dose group included patients treated with lymphoma protocol, RA protocol, 3 or 5 weekly infusions of 375 mg/m2 RTX, and 4 weekly infusions of 500 mg RTX. The low-dose RTX group included patients treated with 2 weekly infusions at a dose of 375 mg/m2 and 2×500 mg infusions 15 days apart. Patients receiving a combined regimen of RTX and IA were grouped independently. Studies reporting the combined use of RTX and IVIG were excluded due to uncommon or extreme dosing regimens of RTX. Patient’s age, sex, disease type, disease duration, disease severity, TDC and TCRon, status of remission, CR duration, relapse, adverse event, and follow-up time were recalculated for these groups.
Definition
Concomitant therapy means a medication used at the same time as RTX infusion. Remission status is defined as in each original study (Table SII1), most of which employed the consensus statement from the International Pemphigus Committee (34). CR was mostly defined as “absence of new lesions with complete healing of old lesions with or without concurrent therapy for at least 2 months”. Severity is regrouped to mild/moderate or severe according to the scoring system in the original study (Table SII1). TDC, TCRon and TCRoff were calculated from the time of the last dose of RTX to the achievement of disease control and CR on or off concurrent therapy. Remission duration of CR was defined as the duration of CR on and off (if possible) until the first relapse or the end of the follow-up period.
Data analysis (See Appendix S11)
Results
Search results and trial characteristics
Of 165 potentially relevant publications identified through the online literature database search, 30 studies including 578 patients with pemphigus fulfilled the inclusion criteria (Fig. 1). The trials included a single randomized comparative study (high-dose vs. low-dose RTX) (21) and 3 comparative studies (13, 16, 22), and these were summarized in Table SI1. One of the 3 comparative studies compared patients treated with RTX vs. conventional therapy (13), and the other 2 studies compared patients undergoing high-dose vs. low-dose RTX (16, 22). The remaining 26 studies were case series. All included studies advocated RTX for the treatment of pemphigus.
Fig. 1. Selection of studies for systematic review and meta-analysis.
Patient characteristics and rituximab use
The clinical and therapeutic data for the 578 patients with pemphigus (82 with PF, and 496 with PV) are summarized in Table SIII1. The mean age and disease duration were 48.2 years and 4.1 years, respectively. The study included 291 (51.8%) male patients, 453 patients were treated with high-dose RTX, 52 with low-dose RTX, and 73 received a combined regimen with IA. The overall means for TDC, TCRon, and remission duration were 1.1, 5.8 and 14.5 months, respectively. The overall rates of CR rate and major adverse events were 75.8% and 3.3%, respectively. The 6-month, 12-month and overall relapse rates were 2% (n = 11/533), 14% (n = 69/484) and 40.2% (n = 214/533), respectively. Subgroup analysis of studies using the International Pemphigus Committee consensus statement (34) showed an overall CR rate of 77.5%. In addition, 38.7% were off all therapies, while 38.8% had CR on minimal therapy after a cycle of RTX-containing regimen. Mean TCRoff was 15.1 months.
Statistical analysis
The pooled ORs of the 3 studies (1 randomized comparative study (21) and 2 comparative studies (16, 22)) for CR (Fig. 2A) and relapse (Fig. 2B) in patients treated with high-dose vs. low-dose RTX were 1.36 (95% CI, 0.54–3.86; p = 0.56) and 0.33 (95% CI, 0.02–4.79; p = 0.42), respectively.
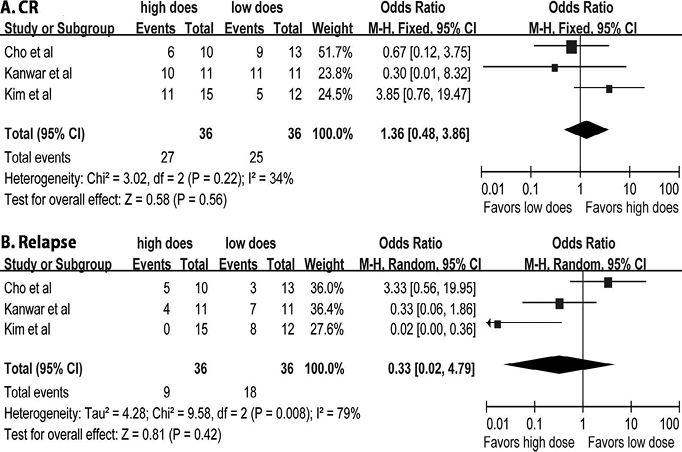
Fig. 2. (A) Odds ratio of complete remission (16, 21, 22). (B) Odds ratio of relapse associated with high-dose vs. low-dose rituximab (RTX) treatment (16, 21, 22). Using a random effects model due to statistical heterogeneity for relapse, with a measure of inconsistency between studies I2 = 79% (p = 0.008). CI: confidence interval; M-H: Mantel-Haenszel methods. For each study: central square = mean effect; line = 95% CI. Large diamond=combined ORs and 95% CIs of overall studies.
Statistical analysis was performed for 213 patients from 17 separate studies (4–10, 12, 13, 16, 17, 21, 23–24, 31, 35, 36), between patients treated with high-dose RTX and low-dose RTX. The univariate analyses of high-dose vs. low-dose and lymphoma vs. RA regimens is seen in Appendix S11. Multivariate logistic regression and linear regression were performed (Table SIV1). After adjustments for age, gender, type of pemphigus, disease duration, disease severity and follow-up time, there was no significant difference between the high-dose and low-dose groups in CR, TDC, TCRon and relapse. However, the high-dose group was significantly associated with longer duration of CR (high-dose vs. low-dose: β coefficient, 2.57; 95% CI: 1.19–4.95) compared with the low-dose group. On the other hand, disease duration was significantly associated with CR (OR 0.90; 95% CI: 0.80–0.97). Longer follow-up time was significantly correlated with longer CR duration (β coefficient 0.47; 95% CI: 0.59–0.64) and a higher rate of relapse (OR 1.11; 95% CI: 1.02–1.20), respectively. The inverse correlation between disease severity and CR duration showed only borderline significance (β coefficient, –2.61; 95% CI: –4.89–0.34; p = 0.07). Multivariate analysis of lymphoma vs. RA regimens is shown in Appendix S11.
Discussion
Current evidence supports the efficacy of RTX in treating patients with pemphigus, with 76% of patients achieving CR. Mean TDC was less than one month, and mean TCRon was 5.8 months after a cycle of RTX. Mean remission duration was 14.5 months, with an overall relapse rate of 40%. In the subgroup analysis by consensus definitions (34), 38.7% were off all therapies, with mean TCRoff of 15.1 months after a cycle of RTX. In a study of patients treated primarily with conventional therapy (CS plus ISA), 77% attained CR, and 51% were off all therapy with a 6-year mean follow-up (37). The mean TCRoff was 36 months using conventional therapy (37). The overall rate of CR using RTX was similar to conventional therapy, but the time to CR off all therapies was shorter for patients treated with RTX, even in refractory or severe cases.
Our analysis showed that high-dose RTX treatment was associated with significantly longer duration of CR and a trend for shorter TDC and lower relapse rate compared with low-dose RTX treatment (Table SIV1). The rates of serious adverse events were similar between the 2 groups. One RCT with 22 patients reported using a significantly lower total dose of ISA and observed a better decline in anti-desmoglein antibodies in the high-dose group, while no differences in TDC or CR were observed between the 2 groups (2 × 1,000 vs. 2 × 500 mg) (21). Kim et al. (22) reported significantly shorter TCR and lower relapse rates for patients in the high-dose group (≥ 3 × 375 mg/m2) compared with patients in the low-dose group (2 × 375 mg/m2), but no statistical difference between the rates of CR. Cho et al. (16) observed no significant difference in outcomes between patients with severe pemphigus treated with high-dose RTX (4 × 375 mg/m2) and mild to moderate severity on low-dose RTX (2 × 375 mg/m2). Disease severity must be taken into account when evaluating the efficacy of RTX. From the previous studies and our analysis, both high-dose and low-dose RTX could eventually lead to CR, but more sustained CR might be reached using higher dose RTX. However, further large-scale RCTs are needed to provide evidence-based data.
The IA-combined protocol resulted in the fastest control of disease before the completion of RTX therapy (Table I). However, there was a trend for a higher rate of serious adverse events (IA-combined vs. high-dose vs. low-dose RTX: 8.5% vs. 2.8% vs. 1.9%; p = 0.06) in the IA-combined group. The combined use of IA remains a promising option if the overall condition of the patient is acceptable and rapid disease control is desired.
Table I. Key issues
| 1. Rituximab is efficacious and well-tolerated in patients with pemphigus. |
| 2. Complete remission rate after 1 cycle of Rituximab was 76%. Mean time to complete remission was 5.8 months, complete remission duration 14.5 months and overall relapse rate 40%. Eighteen patients (3.3%) developed major adverse effects. |
| 3. High-dose (≥ 2,000 mg) Rituximab was associated with longer complete remission compared with low-dose Rituximab (<1,500 mg). |
| 4. No significant difference in time to complete remission, complete remission or relapse rates between the high-dose and low-dose Rituximab. No superiority of lymphoma protocol over rheumatoid arthritis in all outcomes. |
| 5. Immunoadsorption-combined regimens resulted in the fastest control of disease before completion of Rituximab therapy. |
| 6. Choice of optimal regimen may depend on the overall condition of the individual patient. |
In this review, the lymphoma protocol was linked to a trend towards higher CR, shorter TDC and TCRon, and longer remission duration, although there was no significant difference. This seems reasonable due to the higher total dose of the lymphoma protocol compared with the RA protocol. However, in contrast to our study, another review reported a higher CR rate (75%) with the RA protocol compared with the lymphoma protocol (67%) (33). However, the protocols in the previous review did not exclude patients with concomitant IA or IVIG use, and the sample size was smaller. The RA protocol has been increasingly employed because pemphigus is an autoimmune disease that does not require eradication of a malignant B-cell clone as in lymphoma. In addition, the protocol attains an earlier peak RTX concentration and offers the advantages of fewer infusion cycles and lower costs (18–21, 38). Thus, for patients seeking a more rapid response, fewer hospital visits, and lower costs, the RA protocol may provide outcomes equivalent to those with the lymphoma protocol. However, further well-designed long-term studies may be necessary to evaluate the fundamental therapeutic differences between the 2 protocols.
In addition to the influence of the treatment regimen, CR was also significantly influenced by disease duration (Table SIV1). The association between shorter disease duration and higher rate of CR might support the rationale for using RTX as a first-line treatment in pemphigus, even for treatment-naïve patients (12, 19, 24, 31, 39). Lunardon et al. (12) also suggested that treatment with RTX earlier in the course of disease might result in better outcomes. On the other hand, disease severity showed only borderline significance with CR duration. This might be related to the small sample size due to limited use of scoring tools and the difficulty in grouping patients from multiple different scoring systems.
Relapses are not uncommon following RTX therapy. An overall relapse rate of 40% was found in this study, and a longer follow-up time was related to a higher rate of relapse (Table SIV1). Leshem et al. (19) reported a 22% relapse rate with a mean follow-up of 18 months, while an 81% relapse rate was reported by Colliou et al. (35) during a mean follow-up of 74 months. Some patients in the studies with longer follow-up times experienced more than 1 relapse and received repeated cycles of RTX with successful outcomes (9–11, 13, 14, 18, 19, 23, 31, 35).
Study limitations
This study has several limitations. First, most of the studies included were case series. Secondly, the sample size for studies providing complete raw data is rather limited. In addition, disease severity scoring systems for pemphigus (40) varied between studies, making it difficult to stratify disease severity. Few studies provided comprehensive immunological evaluation for analysis, such as desmoglein indices, when there was no determined correlation between B-cell depletion and clinical response (3). Finally, a selection bias might exist with regard to who received treatment and how treatment was administered.
In conclusion, the evidence supports the conclusion that RTX is effective and well-tolerated in the treatment of pemphigus (Table I). Both high-dose and low-dose RTX could lead to CR, while high-dose RTX treatment might provide longer duration of CR. The choice of regimen is based on disease severity, co-morbidities, and the financial constraints of the patient in order to achieve favourable outcomes. However, further larger and controlled studies are needed to establish the optimal dosing schedule and treatment guidelines.
The authors declare no conflicts of interest.

1http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-2116
REFERENCES
