Marion Nadal1,2, Bruno Giraudeau1,3, Elsa Tavernier3, Annie-Pierre Jonville-Bera4, Gérard Lorette1,2 and Annabel Maruani1,2,5
1University François Rabelais Tours, Departments of 2Dermatology and 4Clinical Pharmacology, CHRU Tours, 3Clinical Investigation Center, INSERM 1415, CHRU Tours, and 5INSERM U930, Tours, France
Mammalian target of rapamycin (mTOR) inhibitors are a promising new treatment in vascular anomalies, but no published randomized controlled trials are available. The aim of this systematic review of all reported cases was to assess the efficacy and safety of mTOR inhibitors in all vascular anomalies, except cancers, in children and adults. In November 2014 MEDLINE, CENTRAL, LILACS and EMBASE were searched for studies of mTOR inhibitors in any vascular condition, except for malignant lesions, in humans. Fourteen publications and 9 posters, with data on 25 and 59 patients, respectively, all < 18 years old were included. Of these patients, 35.7% (n = 30) had vascular tumours, and 64.3% (n = 54) had malformations. Sirolimus was the most frequent mTOR inhibitor used (98.8%, n = 83). It was efficient in all cases, at a median time of 2 weeks (95% confidence interval 1–10 weeks). Sirolimus was well tolerated, the main side-effect being mouth sores, which led to treatment withdrawal in one case. The dosage of sirolimus was heterogeneous, the most common being 1.6 mg/m2/day. Key words: mTOR inhibitors; sirolimus; vascular anomalies; vascular tumours; vascular malformations; angioma.
Accepted Nov 24, 2015; Epub ahead of print Nov 26, 2015
Acta Derm Venereol 2016; XX: XX–XX.
Annabel Maruani, Department of Dermatology, CHRU Tours, Hospital Trousseau, Avenue de la République, FR-37044, Tours Cedex 9, Tours, France. E-mail: annabel.maruani@univ-tours.fr
Vascular anomalies include a heterogeneous group of disorders. In 1996, the International Society for the Study of Vascular Anomalies adopted a new classification, distinguishing vascular malformations (VMs) from vascular tumours (VTs) by their clinical, biological, radiological and pathological features (1, 2). The classification was updated in 2014, taking into account newly discovered genetic features (3). VMs result from defective development of the embryonic vascular system and feature dysplastic malformed vessels, which are not always apparent at birth (4). VMs may involve capillaries, veins, arteries, the lymphatic system, or combinations of these (4). They do not regress throughout life; they usually have commensurate growth during childhood and may worsen over time if not treated. VTs are thought to result from endothelial proliferation (5). They are broadly divided into infantile haemangiomas, which are common and autoregressive, and other VTs, which are often more complicated (6).
Treatment of vascular anomalies is largely based on symptoms, and no therapy is a suggested first possibility. Among VMs, capillary malformations might be treated with pulsed dye laser as first-line therapy (7). Venous, lymphatic and arteriovenous malformations have been treated with physical bandages, chemotherapy, sclerotherapy or sclero-embolization, and/or surgery (8–12). Treatment of VTs depends on the tumour subtype. When necessary, infantile haemangiomas are mostly treated with propranolol, whereas other VTs might be treated with steroids, vincristine, interferon, chemotherapeutic agents, radiotherapy or surgery (13–18).
For complicated VMs and VTs, treatments are complex and often disappointing (19). In these cases, mammalian target of rapamycin (mTOR) inhibitors have been found promising (20, 21). In 2008 Marsh et al. (22) first used rapamycin to control life-threatening complications of multiple hamartomas in a patient with Proteus syndrome with germline mutations of phosphatase and tensin homolog (PTEN).
mTOR is a serine/threonine kinase regulated by phosphoinositide-3-kinase (PI3K) and Akt. It acts as a master switch of numerous cellular processes, including cellular catabolism and anabolism, cell motility, angiogenesis, and cell growth (23). Akt was found to be overexpressed in the endothelial cells of cutaneous VMs in a murine model, which activated mTOR (24). mTOR inhibitors directly inhibit mTOR, thereby preventing downstream protein synthesis and subsequent cell proliferation and angiogenesis (23).
The drugs that inhibit the mTOR pathway include sirolimus (rapamycin) and temsirolimus, everolimus and deforolimus, termed rapalogs. Sirolimus was discovered in the early 1970s and developed as an immunosuppressant in the early 1990s (25, 26). It is a macrocyclic lactone produced by Streptomyces hygroscopicus. The drug is available in both liquid and tablet formulations and is currently US Food and Drug Administration (FDA)-approved for preventing kidney allograft rejection in children ≥ 13 years old. However, sirolimus is commonly used in younger children to manage organ rejection. The bioavailability of sirolimus is low (15%), its volume of distribution is large and it has a high hepatic metabolism conferring potential drug interactions (23). The other mTOR inhibitors were developed in the 2000s (27). They are all structurally similar to sirolimus, differing mainly in a single position of the lactone ring (C-40) (23). A commonly stated reason for the development of rapalogs was to improve the pharmacokinetic properties of sirolimus, especially its poor bioavailability and solubility in water.
All mTOR inhibitors have immunosuppressive and anti-neoplastic effects because of their antiproliferative properties. They are used for preventing rejection of kidney transplants (sirolimus, everolimus) and hepatic and cardiac transplants (everolimus) and for treating advanced kidney cell carcinoma and mantle cell lymphoma (temsirolimus), renal cell carcinoma, breast cancer, neuroendocrine tumours of the pancreas, renal angiomyolipoma and subependymal giant cell astrocytoma associated with tuberous sclerosis (everolimus) (28–36). Deforolimus is the last mTOR inhibitor in clinical development (37, 38). It has been tested in clinical trials for sarcoma, endometrial cancer, malignant glioma and haematological malignancies (38, 39).
The aim of this systematic review was to assess the efficacy and safety of mTOR inhibitors in all vascular anomalies in children and adults.
METHODS
Electronic databases were systematically searched for original articles referring to the use of systemic mTOR inhibitors in vascular anomalies. PRISMA guidelines were followed for the systematic review.
Search strategy
The search was performed by one author (MN) with the assistance of an information specialist (OL). Electronic databases MEDLINE via PubMed, CENTRAL, LILACS and EMBASE were searched on 12 November 2014, with no limitations on dates or language. To search for studies of mTOR inhibitors, the following keywords were used: “mTOR inhibitor”, “sirolimus”, “everolimus”, “temsirolimus” and “deforolimus”. To search for all vascular anomalies, the following keywords were used: “angioma”, hemangioma”, “Kasabach-Merritt”, “hemangioendothelioma”, “glomangioma”, “vascular”, “venous”, “capillary”, “lymphatic”, “lymphedema”, “lymphangioma” and “arteriovenous”.
Inclusion and exclusion criteria
Inclusion criteria were: all original reports (study, case series, item of correspondence, posters and meeting abstracts) describing treatment with any systemic mTOR inhibitor, alone or in association, in any vascular condition (VMs and VTs), except for malignant lesions, in humans. Superficial and visceral vascular anomalies were included. Topical treatments were excluded.
Study selection strategy
According to the pre-defined criteria, 2 authors independently and in duplicate (MN, AM) selected reports on the basis of the title, if available, then report abstracts. Any discrepancies were resolved in consultation with a third dermatologist (GL). The 2 authors then examined the full texts of the selected reports. Duplicate publications were identified by several criteria (authors, title, intervention characteristics, and number of patients). In case of duplicates, the most complete report was chosen.
Data extraction
For each selected report, 2 authors (MN, AM) independently extracted information on the first author, publication year, journal, country/site, study design, characteristics of patients, type of vascular anomaly, number of lesions, associated complications, type of mTOR inhibitor, efficacy and side-effects, co-interventions and follow-up. Any disagreements were resolved by discussion. A data table was established for each patient. The extraction table was developed by 3 authors who are dermatologists familiar with vascular anomalies (MN, GL, AM). For missing data, the first author was contacted when possible.
Statistical analysis
A descriptive analysis was performed because the review included only case reports and series. Time to obtain response for VTs, VMs and overall was examined by Kaplan-Meier survival curve analysis on the 25 published cases. Because efficacy criteria were heterogeneous, improvement was recorded if the authors mentioned it, whatever the criteria. A log-rank test was performed to compare the 2 survival curves by using R 2.15.2 (http://www.R-project.org; the R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
From an initial number of 6,076 publications retrieved, 14 full reports and 9 posters were included, corresponding to 25 and 59 patients, respectively (Fig. S11). For the posters, no individual patient data were available.
Characteristics of patients
All patients were < 18 years old. Among vascular anomalies, 35.7% (n = 30) were VTs, mostly kaposiform haemangioendothelioma/tufted angioma, and 64.3% (n = 54) were VMs. For 73 cases (86.9%), vascular anomalies had a lymphatic component (Table I).
Table I. Characteristics of patients and vascular anomalies
|
Total n = 84 n (%) |
Publications n = 25 n (%) |
Posters n = 59 n (%) |
|
|
Sex |
|||
|
Male Female Not reported |
31 (36.9) 36 (42.9) 17 (20.2) |
12 (48.0) 12 (48.0) 1 (4.0) |
19 (32.2) 24 (40.7) 16 (27.1) |
|
Age of onset of sirolimus |
|||
|
< 2 months 2 months–2 years 2–13 years 13–18 years > 18 years Not reported |
3 (3.6) 12 (14.3) 9 (10.7) 3 (3.6) 0 57 (67.9) |
1 (4.0) 7 (28.0) 7 (28.0) 3 (12.0) 0 7 (28.0) |
2 (3.4) 5 (8.5) 2 (3.4) 0 0 50* (84.7) |
|
Vascular anomalies |
|||
|
Congenital Not congenital Not reported |
12 (14.3) 15 (17.9) 57 (67.9) |
10 (40.0) 8 (32.0) 7 (28.0) |
2 (3.4) 7 (11.9) 50 (84.7) |
|
Type |
|||
|
Tumours KHE/TA Haemangiomaa Malformations Arteriovenous Venousb Lymphaticc Venolymphatic Not reported |
30 (35.7) 27 (32.1) 3 (3.6) 54 (64.3) 1 (1.2) 4 (4.8) 38 (45.2) 8 (9.5) 3 (3.6) |
15 (60.0) 13 (52.0) 2 (8.0) 10 (40.0) 1 (4.0) 1 (4.0) 7 (28.0) 1 (4.0) 0 |
15 (25.4) 14 (23.7) 1 (1.7) 44 (74.6) 0 3 (5.1) 31 (52.5) 7 (11.9) 3 (5.1) |
aIncluding one case with PHACE syndrome and one with spindle cell haemangioma in Maffucci syndrome. bIncluding 2 cases with blue rubber bleb naevus syndrome. cIncluding 2 cases with Gorham-Stout syndrome and 15 with kaposiform lymphangiomatosis.
*No individual data were available: median age for 21 cases was 8 years (range 2 months to 13 years); median age for another 21 cases was 13 months (range 2 weeks to 3 years)
KHE: kaposiform haemangioendothelioma; TA: tufted angioma.
All patients had severe symptoms, which were detailed in 44 cases: 6 (13.6%) had functional symptoms (pain, feeding difficulties, decreasing function of a limb, fractures) and 37 (84.1%) had very serious symptoms (gastroenterological bleeding, respiratory distress, Kasabach-Merritt phenomenon).
Treatment
Sirolimus was given in 83 cases (98.8%), everolimus in one case, and deforolimus and temsirolimus for no anomalies. The dosage of sirolimus was heterogeneous, the most common being 1.6 mg/m2/day (16/24 (66.7%) cases with available data). In addition, the expected residual blood level was heterogeneous, the most usual being 5–15 ng/ml (Table II). The time of dosed blood level was specified in one case (15 days). For 14 cases, data on the duration of treatment were reported; for 11 (78.6%), mTOR inhibitors were not withdrawn at last follow-up, which ranged from 1 to 28 months. Sirolimus was stopped gradually in one case.
Table II. Characteristics of the mTOR inhibitors
|
Total n = 84 n (%) |
Publications n = 25 n (%) |
Posters n = 59 n (%) |
|
|
mTOR inhibitors |
|||
|
Everolimus Sirolimus Temsirolimus Deforolimus |
1 (1.1) 83 (98.8) 0 0 |
1 (4.0) 24 (96.0) 0 0 |
0 59 (100.0) 0 0 |
|
Sirolimus starting dosage |
|||
|
< 0.05 mg/kg/day 0.05–0.1 mg/kg/day < 1.0 mg/m2/day 1.5–1.6 mg/m2/day >2.0 mg/m2/day Not reported |
1 (1.2) 4 (4.8) 1 (1.2) 16 (19.0) 2 (2.4) 60 (71.4) |
0 4 (16.0) 1 (4.0) 16 (64.0) 2 (8.0) 2 (8.0) |
1 (1.7) 0 0 0 0 58 (98.3) |
|
Expected blood level |
|||
|
5–15 ng/ml 1–5 ng/ml Not reported |
26 (30.9) 1 (1.2) 57 (67.8) |
19 (76.0) 1 (4.0) 5 (20).0 |
7 (11.9) 0 52 (88.1) |
Data on associated treatments were available for 41 cases; for 27 (65.9%), mTOR inhibitors were associated with other treatments, with few details on dosage. Treatments were steroids, vincristine, propranolol, and co-trimoxazole. The most common associated treatment was steroids and vincristine (n = 7).
Efficacy
Criteria considering efficacy were variable and could be clinical, biological, imaging or combined criteria. Criteria for clinical efficacy consisted of improved appearance of the skin, reduced size of the tumour (the proportion of reduction to consider efficacy was 20–80%), functional improvement and improved vital functions. All these clinical criteria were subjective, based on the assessment of physicians. Criteria of biological efficacy concerned only Kasabach-Merritt phenomenon, improvement consisting mainly in normalization of platelet counts. Imaging was also mentioned for efficacy criteria in a few cases (i.e. reduced tumour size seen on magnetic resonance imaging (MRI)). In this review, mTOR inhibitors were efficient in all cases. The efficacy was obtained at a median delay of 2 weeks confidence interval (CI) 95% (1–10 weeks), range 24 h to 6 months (not shown). The time to improvement did not differ between VTs and VMs (p = 0.241) (Fig. 1).
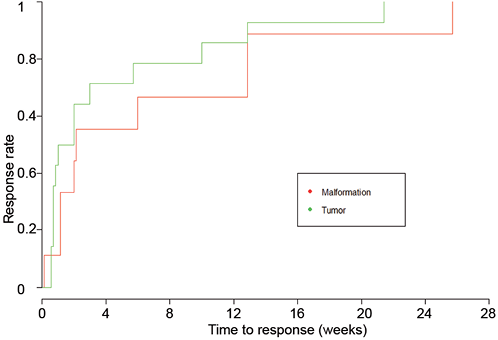
Fig. 1. Survival curves showing time to response rate of sirolimus for vascular tumours and malformations separately in the 25 published cases. This curve shows no significant difference in time to improvement between vascular malformations and tumours.
Safety
In terms of safety of mTOR inhibitors, 6 reports (24 patients, 28.6%) contained no data on adverse effects. When the information was given, no adverse events were noted in 66.7% of cases (n = 40). Regarding clinical side-effects, 12 patients experienced mouth sores (mucositis, stomatitis or oral ulcers), 3 patients experienced infections, 1 patient experienced headaches, and 1 patient experienced hypertension. Among the 3 patients who experienced infections, 1 had a single episode of febrile neutropaenia, 1 had Staphylococcus aureus bacteraemia, and the third had both respiratory syncytial virus infection and influenza, followed a short time later by bacteraemia. Sirolimus was maintained in the 3 cases. Regarding biological side-effects, 9 patients had hypercholesterolemia and 3 showed increased liver enzyme activity. The authors decreased the mTOR inhibitor dosage for 4 cases and stopped it gradually for one case: sirolimus was given at 1.6 mg/m2/day for a diffuse microcystic lymphatic malformation and was withdrawn because of severe oral mucositis.
DISCUSSION
Main results
The aim of this systematic review was to assess the efficacy and safety of mTOR inhibitors for vascular anomalies in children and adults. Data were assessed for 84 patients; all children < 18 years. Sirolimus was the most frequent mTOR inhibitor used and was rapidly efficient in all cases, at a median of 2 weeks 95% CI (1–10 weeks). Sirolimus was well tolerated, the main side-effect being mouth sores, which led to treatment withdrawal in one case.
Comments
The pathophysiology of the mTOR signalling pathway explains the effectiveness of mTOR inhibitors in vascular anomalies. The mTOR protein is a serine-threonine kinase that has a central role in the complex intracellular signalling pathway involved in important processes such as cell growth, cell proliferation, angiogenesis, cellular metabolism, autophagy and apoptosis (Fig. S21). The mTOR complex can be activated by various stimuli via the upstream molecules of insulin, growth factors and hormones (23). Therefore, the inhibitors are effective agents in disorders affecting the mTOR growth control pathway (21).
Studies of mice have demonstrated that constitutive activation of Akt, a serine/threonine-specific protein kinase involved in the upstream mTOR pathway, overexpressed in endothelial cells, led to vascular malformations (24). The PTEN gene encodes a tumour suppressor protein that inhibits the activation of Akt. Mutations in PTEN have been found in several fast- and low-flow vascular anomalies. They were responsible for defective inhibition of Akt and subsequent overexpression of mTOR (40).
Vascular endothelial growth factor (VEGF) is a key regulator in lymphangiogenesis and angiogenesis and acts as both a potential upstream stimulator of, and downstream effector in, the mTOR signalling pathway (21). The mTOR inhibitors decrease VEGF secretion by tumour cells and reduce the sensitivity of endothelial cells to VEGF, which prevents neovascularization. This effect has been demonstrated in vivo and in vitro in murine and human models (41, 42).
In this study, mTOR inhibitors were used for lymphatic malformations and tumours with a lymphatic component (kaposiform haemangioendothelioma and tufted angioma) in 86.9% (n = 73) cases (43). Of note, mTOR inhibitors reproducibly inhibited lymphangiogenesis in 3 independent model systems, including wound healing, embryonic development, and tumour formation and metastasis (44, 45). They inhibit lymphatic neovascularization, decrease the number of lymphatic-vessel endothelial receptors, and inhibit proliferation of human lymphatic endothelial cells by inhibiting VEGF activity (45).
Among the reported mTOR inhibitors in this study, sirolimus was used in all but one case. It was widely used even though it has a lower bioavailability than rapalogs and a higher risk of potential drug interactions, linked to its high hepatic metabolism (23). The reason is probably because it is the oldest mTOR inhibitor drug, and therefore its use is better controlled.
The dosage of sirolimus was heterogeneous, the most common being 1.6 mg/m2/day in 2 doses. For kidney transplants, the loading dose is 3 mg/m2, then 1 mg/ m2 in maintenance. It is used in combination with cyclosporine and corticosteroids during the first 3 months, cyclosporine being an enzyme inhibitor that induces greater absorption of sirolimus and therefore greater blood concentration. Hence, the maintenance dose is less than that found in our patients. The expected blood levels of sirolimus were heterogeneous, but most were 5–15 ng/ml. In kidney transplantation with sirolimus, the most validated expected blood level is somewhat similar: 4–12 ng/ml in initiation treatment and 12–20 ng/ml in maintenance treatment (46).
Classically described adverse effects of sirolimus include mucositis, headaches, asthenia, gastrointestinal effects, peripheral oedema, hypertension and defect healing. Renal dysfunction, especially proteinuria, has also been reported in transplant and non-transplant patients exposed to mTOR inhibitors (47). The most common biological effects are haematological (thrombocytopaenia, leucopaenia, anaemia, microcytosis) and metabolic effects (hyperlipidaemia, hyperglycaemia, hypokaliemia, hypophosphataemia and increased levels of liver enzymes) (46–50). A very rare and potentially serious side-effect is interstitial pneumonitis (51). Finally, given the potential risk of immunosuppression, some authors systematically prescribe co-trimoxazole or pentamisine for Pneumocystis prophylaxis (21).
Study limitations
The first limitation of this study is that only case reports and no trial reports were found, thus the results are difficult to interpret in the absence of reference groups and no meta-analysis can be performed. Secondly, this systematic review showed 100% efficacy of mTOR inhibitors in vascular anomalies, whether VTs or VMs. This efficacy is probably linked to publication bias (i.e. only successful treatment is reported and failures are not). Thirdly, the heterogeneity of patients and conditions make comparisons difficult. Also, the heterogeneity of criteria to assess the efficacy of treatments hinders interpretation of the response rate. Finally, some data were not reported, especially in abstracts.
Conclusion
mTOR inhibitors, especially sirolimus, appear to be efficient for vascular anomalies in children, with variable doses, the most common being 1.6 mg/m2/day. However, the appropriate dosing schedule, adjusted to body weight and age, and the optimal blood levels of sirolimus to obtain therapeutic efficacy with the lowest rate of adverse effects for vascular anomalies need to be defined. Randomized controlled trials are needed to determine the efficacy and safety of these drugs.
ACKNOWLEDGEMENTS
The authors would like to thank Patricia Casanova and Olivier Lauze for their technical assistance.
The authors declare no conflicts of interest.

1http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-2300
REFERENCES
