Gabriel Costa de Carvalho, Rosana Domingues, Marcelle Almeida de Sousa Nogueira, Anna C. Calvielli Castelo Branco, Kelly C. Gomes Manfrere, Naiura Vieira Pereira, Valeria Aoki, Mirian Nacagami Sotto, Alberto J. Da Silva Duarte and Maria Notomi Sato
Laboratory of Dermatology and Immunodeficiency (LIM-56), Department of Dermatology, Medical School, University of São Paulo, São Paulo, SP, Brazil
Lichen planus (LP) is a chronic inflammatory mucocutaneous disease. The inflammatory status of LP may be related to S100A8 (myeloid-related protein 8; MRP8) activation of cytotoxic cells. The aims of this study were to evaluate S100A8 expression in skin lesions and the in vitro effects of S100A8 on CD8+ T cells and natural killer (NK) cells in LP. Increased levels of S100A8/S100A9 were detected in the skin lesions as well as in the sera of subjects with LP. S100A8 expression induced an increased cytotoxic response by peripheral blood CD8+CD107a+ T cells as well as by NK CD56bright cells in patients with LP. Increased expression of interleukin (IL)-1β, tumour necrosis factor (TNF) and IL-6 in the CD8+ T cells of patients with LP was induced by S100A8, in contrast to the control group that produced IL- 10 and interferon type I genes. These data suggest that, in individuals with LP, S100A8 may exert distinct immunomodulatory and cytotoxicity functions. Key words: lichen planus; S100A8; CD8+ T cells; natural killer cells; Toll-like receptor.
Accepted Dec 2, 2015; Epub ahead of print Dec 3, 2015
Acta Derm Venereol 2016; XX: XX–XX.
Maria Notomi Sato, Laboratory of Dermatology, University of Sao Paulo School of Medicine, Av. Dr. Enéas de Carvalho Aguiar, 470, 3o. Floor IMT II, Cerqueira César, São Paulo, CEP-05403-002, Brazil. E-mail: marisato@usp.br
Lichen planus (LP) is a chronic inflammatory skin disease that most frequently involves the skin and the oral mucosa (1, 2). Although the aetiopathogenesis of LP is unclear, several possible causes, including genetic predisposition and environmental triggers, can lead to a continuous inflammatory response. Viral infections, such as human herpes virus type 7 (3) or human hepatitis C virus (HCV), may also be associated with LP (4).
Endogenous danger signals (danger-associated molecular patterns [DAMPs] as well as exogenous pathogen-associated molecular patterns [PAMPs]) may activate cytotoxic T lymphocytes, causing the destruction of keratinocytes in the skin of patients with LP (5). The autoreactivity of CD8+ T cells and the development of systemic autoimmunity in a mouse autoimmune model have been shown to be related to the local production of DAMPs, including the myeloid-related proteins S100A8 and S100A9, which bind to the Toll-like receptor TLR4 and lead to increased interleukin (IL)-17 expression (6). The elevated sera levels of S100A8 and S100A9 proteins are observed in numerous pathological conditions associated with inflammation in humans (e.g. rheumatoid arthritis, systemic lupus erythematosus, diffuse cutaneous systemic sclerosis, psoriasis and cancer (7–12)). We have observed previously that peripheral blood mononuclear cells (PBMC) from individuals with LP are hyper-responsive to activation through the TLR2, TLR4 and TLR5 pathways, with increased secretion of tumour necrosis factor alpha (TNF-α) (13). Therefore, altered innate immunity could contribute to the inflammation and T-cell autoreactivity in LP.
Moreover, S100A8/A9 proteins induce the production of proinflammatory and pro-angiogenic cytokines, which attract immune cells to the psoriatic skin lesions (14). The inflammatory skin milieu can attract CD8+ T cells as well as natural killer (NK) cells in LP, psoriasis and cutaneous lesions of atopic dermatitis (15). The NK cell CD56bright subtype, which predominates in the inflammatory sites of LP lesions, is able to produce perforin, interferon (IFN)-γ and TNF-α (16). It is currently unknown whether altered serum levels of S100A8/A9 in LP can modulate the cytotoxic functions of NK cells or CD8+ T cells.
This study investigated the levels of S100A8 in the skin lesions and sera of patients with LP as well as the immunomodulatory S100A8 on CD107a molecules, which are indicators of degranulation, and cytokine secretion by CD8+ T cells and the NK CD56+ cell subtype from peripheral blood. The findings demonstrate increased S100A8/A9 expression in the skin lesions and sera of patients with LP, which may positively regulate the cytotoxic responses of NK and CD8+ T cells. Moreover, the effects of S100A8 on CD8+ T cells in patients with LP involved the up-regulation of pro-inflammatory cytokine gene expression in association with altered TLR expression.
MATERIALS AND METHODS (see Appendix S11)
RESULTS
Expression of S100A8 in skin lesions of patients with lichen planus
The histology and expression of the S100A8 protein was evaluated in skin biopsies of LP (n = 12) and healthy controls (HC, n = 12). Compared with the epidermal and dermal layers of H&E-stained HC samples (Fig. 1a) LP samples showed increased epidermal cell layers with degeneration of basal cells and an intense cellular infiltration in the superficial dermis (Fig. 1b). S100A8 staining demonstrated intense protein expression in epidermal keratinocytes and diffuse labelling in the superficial dermis (Fig. 1d), contrasting the scarce S100A8 expression in HC samples (Fig. 1c). The combined results of all 12 samples show increased S100A8 protein expression in both the epidermis (Fig. 1e) and dermis (Fig. 1f) of subjects with LP compared with the skin of healthy individuals.
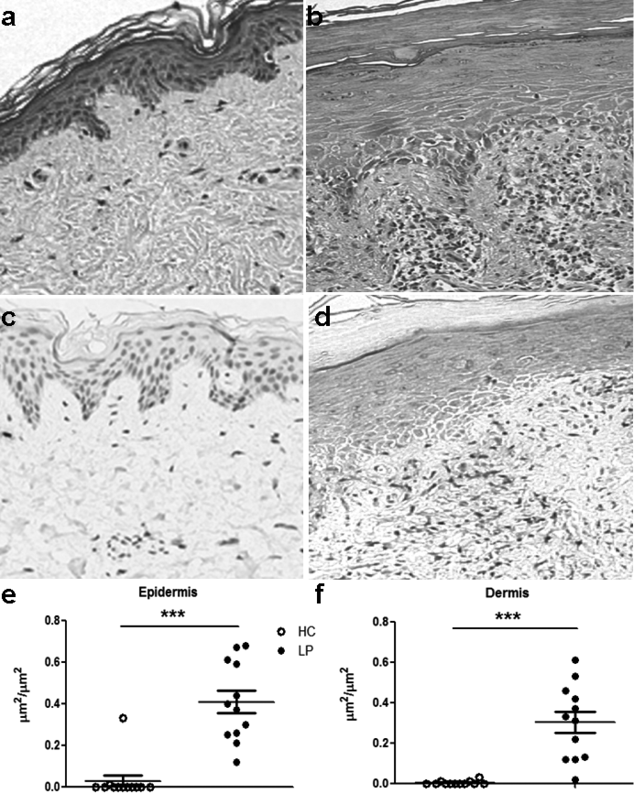
Fig. 1. Enhanced expression of S100A8 in the epidermis and dermis of skin lesions from patients with lichen planus (LP). Skin biopsies from (a, c) healthy subjects and lesions from (b, d) patients with LP were stained with haematoxylin and eosin; (a, b) 100× magnification. (c, d) S100A8 protein levels were measured using immunohistochemistry. The distribution of S100A8 in the (e) epidermal and (f) dermal layers of healthy control skin (HC, n = 12) or in the lesions of patients with LP (n = 12) was evaluated. Calculation of the S100A8-positive area in the dermis was performed by dividing the area positive for the S100A8 protein by the total area. The data are presented as means ± standard error of the mean (SEM). ***p < 0.0001.
Analysis of S100A8 mRNA expression in the skin and S100A8 serum levels in patients with lichen planus
To further evaluate S100A8/S100A9 expression, we assessed S100A8 and S100A9 transcripts in skin lesions from subjects with LP and in HC skin using real-time quantitative qPCR. Higher S100A8 and S100A9 mRNA expression was detected in the LP skin samples compared with the HC samples (Fig. 2a, b). In addition, a highly positive correlation between S100A8 and S100A9 transcript levels was verified in the skin, suggesting that S100A8/S100A9 dimers may form (Fig. 2c, d).
Because we had verified that S100A8/S100A9 expression is up-regulated in the skin cells of subjects with LP, we next evaluated the circulating serum levels of this protein using ELISA. Fig. 2e shows that the levels of this protein were significantly increased in the sera of subjects with LP (median 1,766.1 ng/ml, range 602.2–4,911.4 ng/ml) compared with the control group (median 967.8 ng/ml, range 111.5–2,764 ng/ml).
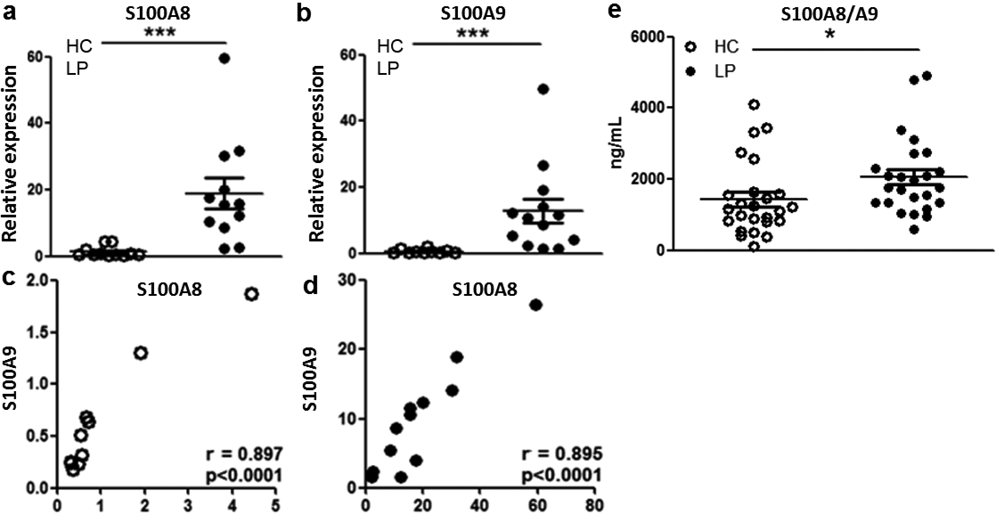
Fig. 2. Up-regulation of S100A8 and S100A9 expression in the skin and increased S100A8 and S100A9 levels in the sera of patients with lichen planus (LP). (a, b) Skin biopsies from healthy controls (HC, n = 12) or lesions of patients with LP (n = 12) were analysed for the expression of S100A8 and S100A9 mRNA using real-time PCR (relative to housekeeping gene). Correlation between S100A8 and S100A9 mRNA expression in the skin in (c) HC and (d) subjects with LP. (e) Levels of the S100A8/S100A9 dimer in the sera of LP (n = 25) and HC (n = 21) were evaluated using enzyme-linked immunoassay (ELISA). The data are presented as means ± standard error of the mean (SEM). ***p < 0.0001, *p ≤ 0.05.
In vitro effects of S100A8 on cytotoxic cells after stimulation via TLR4
The effects of S100A8 expression on the cytotoxic responses of CD8+ T cells and NK cells were then evaluated using the degranulation marker CD107a after stimulating PBMCs; both S100A8 and lipopolysaccharide (LPS) are agonists of TLR4. Phorbol 12-myristate 13-acetate (PMA) and ionomycin were used to stimulate the cells. The gating strategy for CD8+ T cells is shown in Fig. S11, and for NK cells in Fig. S21.
There was an increased baseline frequency of unstimulated CD8+ CD107a+ T cells in subjects with LP compared with HC (Fig. S3a1). Stimulation with LPS or S100A8 increased the frequency of CD8+CD107a+ T cells in subjects with LP compared with the HC group. However, simultaneous stimulation with LPS and S100A8 did not alter the frequency of CD107+CD8+ T cells (Fig. S3a1).
In addition, an increased baseline frequency of TNF+CD8+ T cells was found in subjects with LP compared with HC (Fig. S3b1). Moreover, IL-1β secretion by CD8+ T cells was elevated following simultaneous stimulation with LPS and S100A8 (Fig. S3c1). The synergistic effect of LPS and S100A8, which both act through TLR4, increased the pro-inflammatory response by CD8+ T cells in subjects with LP.
We next evaluated CD107a, TNF and IL-1β expression in response to the TLR4 agonists LPS and S100A8, or to PMA and ionomycin, in CD56dim and CD56bright NK cells. The increased frequency of NK CD56brightCD107a+ cells following S100A8 stimulation was verified in LP compared with HC (Fig. S4a1). Conversely, NK CD56dimTNF+ cells from the LP group were more responsive to PMA and ionomycin stimulation than the HC group (Fig. S4b1). Only low levels of IL-1β in NKCD56dim and NKCD56bright cells were induced after stimulation, and these levels did not differ between the analysed groups (Fig. S4c1).
Profile of Toll-like receptor pathway gene expression in CD8+ T cells stimulated with S100A8
Given our observation that circulatory CD8+ T cells exhibit distinct activation in patients with LP, CD8+ T cells were sorted from PBMCs to evaluate the direct effects of S100A8 on genes related to innate immunity and the TLR signalling pathway. Table I shows the up-regulated and down-regulated TLR pathway gene expression after S100A8 stimulation in LP and HC T cells.
Table I. Differentially regulated genes in the Toll-like receptor (TLR)-mediated signalling pathway of CD8+ T cells after stimulation with S100A8 between the lichen planus (LP) and healthy control groups
|
Lichen planus |
Healthy controls |
|||
|
Gene symbol |
Fold regulation |
Gene symbol |
Fold regulation |
|
|
IL1B |
4.8929 |
IFNB1 |
3.1207 |
|
|
IL6 |
2.4921 |
IL10 |
4.1753 |
|
|
PTGS2 |
2.3577 |
TLR7 |
3.6264 |
|
|
TLR3 |
4.5024 |
CCL2 |
–8.5434 |
|
|
TLR5 |
2.6342 |
CD180 |
–3.7619 |
|
|
TLR8 |
3.3652 |
CD80 |
–3.2825 |
|
|
TNF |
2.1795 |
CD86 |
–2.126 |
|
|
BTK |
–2.06 |
CLEC4E |
–4.9524 |
|
|
CD86 |
–4.3049 |
IFNA1 |
–2.1016 |
|
|
CLEC4E |
–6.8811 |
IL8 |
–2.2317 |
|
|
CSF3 |
–3.8798 |
|||
|
IFNA1 |
–3.7304 |
|||
|
TLR4 |
–2.0984 |
|||
|
TLR7 |
–3.2102 |
|||
Fold regulation of scatterplot analysis of up-regulated (italic) and down-regulated (bold) genes of CD8+ T cells stimulated with S100A8 compared with unstimulated CD8+T cells from subjects with LP (left) and HC group (right).
Consistent with this, Fig. 3 shows up-regulation of the regulatory cytokine IL-10 and genes related to the type I IFN response, such as IFNB1 and TLR7, in CD8+ T cells of HC and patients upon S100A8 stimulation. It is notable that subjects with LP did not up-regulate these regulatory cytokines upon S100A8 stimulation; instead, this group overexpressed inflammatory genes such as IL-1β, TNF and IL-6. Contrasting effects were also observed with TLR expression: although TLR5 and TLR8 expression in CD8+ T cells was down-regulated upon S100A8 stimulation in the HC group, expression was up-regulated in the LP group, and the opposite effects were observed for TLR7 gene expression. Moreover, the data showed that although one of the S100A8 receptors could be TLR4, this DAMP is able to modulate the expression of other TLRs.
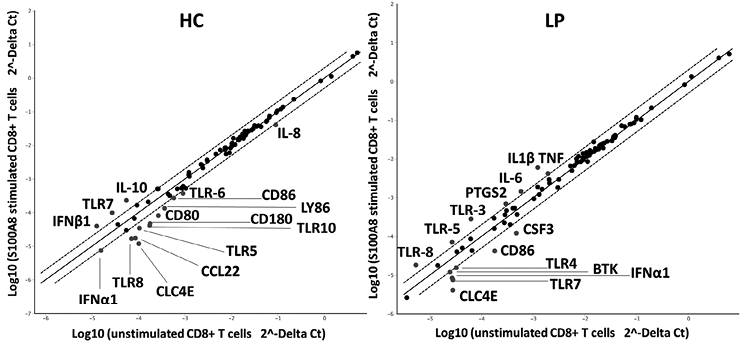
Fig. 3. Influence of S100A8 on the expression of genes related to the Toll-like receptor (TLR)-signalling pathway in CD8+ T cells. Total mRNA from CD8+ T cells from healthy controls (HC) (n = 3) and patients with lichen planus (LP) (n = 3) was isolated after 4 h of stimulation with S100A8. Quantitative real-time PCR with the Human Toll-Like Receptor Signalling Pathway RT2 profiler PCR array was performed. Analysis using Student’s t-test was performed to determine significant gene expression fold changes upon stimulation with S100A8 compared with basal levels in the HC and LP groups. Dots represent the means from 3 independent donors. Genes that were significantly up-regulated by more than 2-fold are marked with their gene name.
DISCUSSION
In the present study, we observed increased expression of S100A8 and S100A9 transcripts in the skin lesions of subjects with LP as well as increased serum levels of the complex. The use of recombinant S100A8 in PBMC culture revealed differences in the cytotoxic responses of NK cells as well as TCD8+ cells between the groups. These results suggested that S100A8 maintained cytotoxic activity in LP. Moreover, S100A8 directly activated TCD8+ cells, leading to pro-inflammatory cytokine expression in LP (IL-6, TNF, IL-1β), whereas in HC, the regulatory cytokine IL-10 was expressed. Although the S100A8 receptor could be TLR4, CD8+T cells activated by S100A8 induced altered TLR expression, including TLR3, TLR5, TLR7, and TLR8, which were differentially expressed between the LP and HC groups. This fact shows that the antiviral and antimicrobial responses are mediated in different ways in the presence of S100A8 between groups. It seems that S100A8 induced compensation in the expression of TLRs; while some are up-regulated others could be inhibited, in dependency to their baseline levels. Moreover, in healthy individuals S100A8 down-regulated TLR5 as well as TLR8 expression, probably to avoid the pro-inflammatory responses, differently from LP CD8+ T cells.
We demonstrated an intensification of S100A8 transcripts and protein expression levels in the skin lesions of subjects with LP, especially in keratinocytes, as already shown by Kunz et al. (19). The increased serum levels of S100A8/A9 may also induce NK and CD8+ T-cell migration to skin lesions and may be associated with an enhancement of their cytotoxic and cytokine responses, which, in turn, leads to the destruction of keratinocytes. Elevated levels of the S100A8 and S100A9 proteins are also a hallmark of numerous pathological conditions that are associated with inflammation (20–22). Indeed, S100A8 affected the up-regulation of pro-inflammatory cytokine gene expression in CD8+ T cells from subjects with LP. Moreover, activation of PBMCs with S100A8 maintained the differential frequencies of CD8+ CD107a+ T cells from subjects with LP compared with HC, and simultaneous activation with LPS and S100A8 increased the production of IL-1β by CD8+ T cells in subjects with LP. S100A8 is a major calcium-binding protein that can act as a second messenger after LPS activation, thereby inducing assembly of the inflammasome platform. In fact, S100A8 and S100A9 mediated the activation of NFκB, the NoD-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) protein, and pro-IL-1β in PBMCs (23).
The pro-inflammatory response induced by S100A8 in LP was observed via the up-regulation of the IL-6, TNF and IL-1β genes in lieu of the regulatory cytokine IL-10 observed in the HC group. IL-10 exerts an inhibitory role in the human monocyte synthesis of IL-1β, IL-6 and TNF after LPS activation (21). IL-10 production by CD8+ T cells after S100A8 stimulation was detected only in HC, suggesting a defect in regulatory cytokine secretion in LP individuals. Our previous results showed that LPS induced decreased IL-10 secretion by PBMCs in individuals with LP (13). In addition, it must be further determined whether the CD8+ T cells secreting IL-10 are regulatory CD8+ T cells or Tc2 cells. Altogether, our results showed that TLR4 induction in LP leads to decreased IL-10 secretion, which probably contributes to the maintenance of pro-inflammatory status conferred by IL-1β, IL-6 and TNF gene expression.
The down-regulation of IFN-α in CD8+ T cells in LP may suggest that S100A8 cannot control the viral response in individuals with LP. S100A8 mediates the anti-viral response in the HC group by inducing the up-regulation of IFN-1β and TLR7 gene expression. We have previously observed that subjects with LP have an impaired IFN-α response in PBMCs stimulated with a TLR7 agonist (imiquimod), which is restored by the activation of TLR7/TLR8 (CL097) (13). Indeed, we found that CD8+ T cells from patients with LP activated with S100A8 exhibited the up-regulation of TLR8, but the down-regulation of TLR7. We also detected the down-regulation of TLR4 after stimulation with S100A8 in human LP CD8+ T cells. The decreased TLR4 expression after S100A8 stimulation of the CD8+ T cells of subjects with LP suggests an interaction between S100A8 and this receptor.
We verified that S100A8 is able to enhance the degranulation of CD56bright NK cells from patients with LP. It is possible that this subtype of NK cells migrates to sites of skin injury, given that CD56bright NK cells could be found in skin lesions of cutaneous LP (24). However, it remains to be determined whether the NK cells that migrate to the skin acquire the CD56bright NK profile by influencing the microenvironment.
Altogether, our findings highlight the role of S100A8 as an important DAMP that could regulate cytotoxic activity locally at skin lesions as well as systemically in patients with LP. This study provides evidence for the immunomodulatory role of S100A8 as well as the cytotoxicity functions mediated by NK and CD8+ T cells in individuals with LP.
ACKNOWLEDGEMENTS
The authors are grateful to all individuals who participated in the study. This work was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (2011/20740-3) and the Laboratório de Investigação Médica, Unidade 56 do Hospital das Clínicas da Faculdade de Medicina de São Paulo.
The authors declare no conflicts of interest.

1http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-2306
REFERENCES
