Danique A. van der Krieken1#, Thomas H. A. Ederveen2#, Sacha A. F. T. van Hijum2,5, Patrick A. M. Jansen1, Willem J. G. Melchers3, Paul T. J. Scheepers4, Joost Schalkwijk1 and Patrick L. J. M. Zeeuwen1
1Department of Dermatology, 2Centre for Molecular and Biomolecular Informatics, 3Department of Medical Microbiology, and 4Department for Health Evidence, Radboud Institute for Molecular Life Sciences and Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, and 5NIZO Food Research, Ede, The Netherlands
#These authors contributed equally to this work.
The diversity and dynamics of the skin microbiome in health and disease have been studied recently, but adequate model systems to study skin microbiotas in vitro are largely lacking. We developed an in vitro system that mimics human stratum corneum, using human callus as substrate and nutrient source for bacterial growth. The growth of several commensal and pathogenic bacterial strains was measured for up to one week by counting colony-forming units or by quantitative PCR with strain-specific primers. Human skin pathogens were found to survive amidst a minimal microbiome consisting of 2 major skin commensals: Staphylococcus epidermidis and Propionibacterium acnes. In addition, complete microbiomes, taken from the backs of healthy volunteers, were inoculated and maintained using this system. This model may enable the modulation of skin microbiomes in vitro and allow testing of pathogens, biological agents and antibiotics in a medium-throughput format. Key words: skin; callus; bacteria; microbiome; antibiotics.
Accepted Mar 10, 2016; Epub ahead of print Mar 15, 2016
Acta Derm Venereol 2016; 96: XX–XX.
Patrick L. J. M. Zeeuwen and J. Schalkwijk, Department of Dermatology, Radboud University Medical Center, PO Box 9101, NL-6500 HB Nijmegen, The Netherlands. E-mail: Patrick.Zeeuwen@radboudumc.nl; Joost.Schalkwijk@radboudumc.nl
The skin is a physical barrier that protects the interior of the human body from the exterior. It supports a complex microbial ecosystem that is in homeostasis with its host (1). The microbial communities residing on the skin have been investigated recently using culture-independent methods (2, 3). These communities, termed microbiotas, are considered beneficial for our health (4). Furthermore, disturbance of “normal” microbial communities, a condition called “dysbiosis”, in which homeostatic relations between the host and its microbiota are disturbed, is associated with skin diseases (5). Skin microbiotas are therefore being studied in a search for markers related to disease onset, progression, and outcome, which may lead to the development of novel therapies for skin diseases.
Different factors influence the microbiota composition of the skin; for example, lifestyle, host demographic and environmental characteristics, the use of antibacterial agents, and health status (6–9). Skin alterations and dysregulated immune responses could cause shifts in the microbiota composition (10–14) and, on the other hand, skin microbiotas can modulate cutaneous immunity (15–17). The microbial diversity of lesional skin is altered in inflammatory skin diseases, such as psoriasis and atopic dermatitis (12, 18–20). However, it is not known if these shifts in bacterial composition have a causal role in disease or are merely a consequence of disturbed skin homeostasis.
Changes in bacterial diversity, microbiota composition, and host-defence interactions in health and disease can be studied using skin models. For instance, human reconstructed skin models and ex vivo skin models have been used to study the efficacy of antibacterial compounds (21–24) or the colonization and infection characteristics of certain pathogens (25–29). However, these models are laborious, expensive, prone to bacterial overgrowth, and their throughput is low.
Under normal skin conditions, most of the bacteria reside in the upper half of the stratum corneum (10), feeding on nutrients derived from the corneocytes, which consist mainly of cross-linked protein and lipids. The current study investigated whether human callus could serve as a substitute for stratum corneum to support the growth of skin commensals and pathogens, for use as a model for investigating bacterial growth, and assessing the efficacy of antimicrobial compounds.
MATERIALS AND METHODS
Callus collection and preparation
Human callus from the heels of 3 healthy volunteers was collected using a callus rasp (Ped Egg™). The callus was mixed, frozen in liquid nitrogen, then ground up using a Micro Dismembrator U (B. Braun Biotech International, Melsungen, Germany), as described previously (30). Phosphate-buffered saline (PBS; Fresenius Kabi GmbH, Graz, Austria) was added to the callus powder to create a 2% suspension, which was sterilized by exposure to gamma radiation (16.2 kGy/63 h). The sterilized callus suspension was stored at 4°C or –20°C until further use.
Preparation of the in vitro skin model
Sterile agar (2% in PBS; Becton, Dickinson and Co., Sparks, MD, USA) was added to wells of 24-well (1 ml agar) and 96-well (100 µl agar) cell culture plates. A 100 µl (24-well) or 20 µl (96-well) volume of sterile callus suspension (2% in PBS) was applied on top of the agar. The plates were allowed to dry for 24 h at 37°C, and stored at 4°C until further use. For schematic representation see Fig. 1.
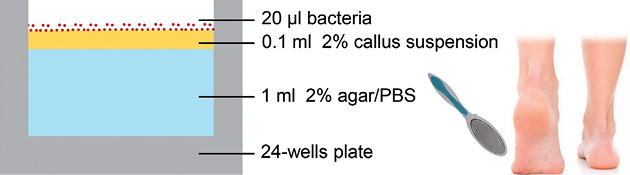
Fig. 1. Structure of the human cutaneous microbial ecosystem model. The model is prepared in a 24-well plate. A 2% callus suspension, originating from the heel of healthy volunteers, on top of 2% agar (in phosphate-buffered saline) is the basis of the model. After drying, bacteria are inoculated on top of the dead corneocytes and cultured at 32°C.
Bacterial cultures
Staphylococcus epidermidis (ATCC 12228), Propionibacterium acnes (ATCC 6919), Staphylococcus aureus (ATCC 29213), Pseudomonas aeruginosa (ATCC 27853) and Streptococcus pyogenes (ATCC 12344) strains were obtained from the Department of Medical Microbiology of the Radboud University Medical Center. Bioluminescent S. aureus (Xen36) was purchased from Caliper Life Sciences (Boston, MA, USA). Bacteria were inoculated on Columbia agar with 5% sheep blood (Becton, Dickinson and Co.) overnight at 37°C. A single colony of each plate was picked and cultured in brain heart infusion medium (Mediaproducts BV, Groningen, The Netherlands) overnight at 37°C, except for P. acnes, which was cultured in thioglycollate medium (Becton, Dickinson and Co.) under anaerobic conditions for 2 days at 37°C. Bacterial suspensions were diluted 10 times in medium and allowed to grow for another 3 h to reach exponential bacterial growth (except for P. acnes). Thereafter, the bacteria were collected by centrifugation, washed twice with PBS and finally resuspended in PBS, resulting in bacterial concentrations of 104~1012 colony-forming units (CFU)/ml. Bacterial suspension aliquots, 20 µl or 5 µl, were added to each well of the callus model (in 24-well and 96-well plates, respectively). When a mixture of bacterial strains was examined on the model, the same total number of CFU of every strain was used. The bacteria on the model were incubated at 32°C for different time-points for up to one week.
Counting colony-forming units
The entire model (agar + callus + bacteria) was lifted out of the 24-well plate and transferred to a 50 ml tube containing 10 ml PBS (Fig. S11). The tubes were vortexed at maximum speed for 1 min to detach and suspend the bacteria. The aqueous solution containing the bacteria (including some callus particles, but without the agar) was transferred to a new tube. These samples were serially diluted in steps of 10. Ten µl of each dilution was placed on sheep blood agar plates and incubated overnight at 37°C. Next day, visible colonies on the plate were counted for each dilution.
Microbiome samples
Microbiome samples from the lower backs of 2 male volunteers were collected by swabbing the skin with Sample Collection Swabs (Epicentre Biotechnologies, Madison, WI, USA), as described previously (10). The swabs were collected in PBS, centrifuged for 5 min at 5000 rpm, resuspended in 60 µl PBS and divided over the model in 20 µl fractions. The model was incubated at 32°C for 1 week. Samples were collected for propidium monoazide (PMA)-quantitative PCR (qPCR) analysis and PMA-Illumina sequencing on days 0, 1 and 7.
Antibiotics
Tetracycline (Sigma-Aldrich, Zwijndrecht, The Netherlands) was added in different concentrations to the agar (2% agar in PBS). Callus and bacterial suspensions were added to the model as described above. Bacterial survival was assessed by counting CFU after culturing for 24 h.
Propidium monoazide treatment
PMA treatment of collected bacteria was performed to eliminate microbial genomic DNA (gDNA) originating from non-viable cells. Bacteria were collected from the model and resuspended in 500 µl PBS to which 1.25 µl PMA (20 mM, Biotium, Hayward, CA, USA) was added. These mixtures were incubated for 10 min in the dark, and exposed to light for 5 min using PhAST Blue equipment (GenIUL, Terrassa, Spain). The samples were then centrifuged for 5 min at 5,000 rpm, and the pellet resuspended in 300 µl Micro Bead solution (MO BIO Laboratories, Carlsbad, CA, USA). To obtain “non-viable” cells, bacteria were heat-killed at 85°C for 45 min. The viability of these bacteria was then assessed by culturing on blood agar plates (Becton, Dickinson and Co.).
Microbial genomic DNA isolation
Microbial gDNA was extracted using the MO BIO Ultraclean Microbial DNA Isolation Kit (MO BIO Laboratories) with modifications, as described previously (10). Microbial gDNA samples were stored at –20°C until further processing.
Quantitative PCR
Microbial gDNA was used as template for qPCR amplification with SYBR Green using the Bio-Rad CFX Connect apparatus (Bio-Rad, Hercules, CA, USA). Details of qPCR and the design of strain-specific primers is given in Appendix S11.
16S amplification prior to sequencing
Microbiota samples derived from the skin of the lower back contained small amounts of microbial gDNA. Therefore, we introduced a pre-amplification step of the V3–V6 regions of the 16S rRNA gene, as reported previously (10). Details of amplicon generation and used primers are given in Appendix S11.
16S metagenomic library preparation and Illumina sequencing
Illumina 16S metagenomic amplicon libraries were generated and sequenced at BaseClear BV (Leiden, The Netherlands). A brief description of the procedure is given in Appendix S11.
Sequencing data analysis
Demultiplexed FASTQ files, as provided by BaseClear, were first used to generate Illumina paired-end sequence pseudoreads by PEAR (31) using the default settings. The resulting pyrosequencing data were analysed with a customized QIIME v1.2 (32) workflow, as described previously (10). Hierarchical (UPGMA) clustering was performed with weighted UniFrac as its distance measure; figures resulting from these clustering analyses were generated using the interactive tree of life (iTOL) tool. The PD whole tree alpha diversity was calculated by bootstrapping 43,592 reads per sample, and taking the mean over 4 bootstrap trials.
Bioluminescent Xen36 analysis
The bioluminescence of the S. aureus Xen36 strain on the model was detected with In Vivo Imaging System (IVIS; PerkinElmer, Waltham, MA, USA) apparatus, and analysed with Living Image 3.1 software (PerkinElmer, Waltham, MA, USA).
RESULTS
Development and validation of the model: callus as a source of nutrients
In vivo, bacteria live and attach to the dead corneocytes of the outer layers of human skin. Therefore, we hypothesized that callus would be a natural source of nutrients on which microorganisms could survive and grow. The model (Fig. 1) was prepared in a 24-well plate, using agar (devoid of nutrients) as a basis for callus suspension and to maintain a sufficiently hydrated surface. After drying the applied callus suspension, a thin layer of dead corneocytes is present on top of the agar, upon which the bacteria are inoculated and cultured. The procedure for collection of the bacteria from the wells and subsequent analysis by determination of CFU is shown in Fig. S11.
Regulation of the proliferation rate of the bacteria: dynamic vs. static model
In vivo, the number of bacteria on healthy human skin remains at a steady-state level, meaning that proliferation rates are low and no overgrowth, as in overt infection, occurs. To mimic these conditions in vitro, a static model is required. However, if an infectious situation needs to be mimicked, a more dynamic model is preferred. This could be useful, for instance, when antimicrobial compounds are tested. We therefore inoculated the model with different numbers of 2 skin commensals (S. epidermidis and P. acnes) and 2 relevant skin pathogens (S. aureus and P. aeruginosa), cultured them at 32°C, and determined the correlation between the starting concentration and the growth of these bacteria. The samples were analysed on days 1, 4 and 7 by counting CFU (Fig. 2). On day 1 CFU/ml was comparable for all samples, except for P. acnes, regardless of the different starting concentration of the inocula. It was shown that the bacteria that were applied at a lower concentration proliferated more rapidly than those applied at higher concentrations. The 2 highest concentrations of P. acnes proliferated 102–103 times from day 0 to day 1, the number of CFU/ml at lower concentrations decreased at every time-point (Fig. 2b). From day 1 to day 7, the level of S. epidermidis remained steady (108–1010 CFU/ml) for all different starting concentrations (Fig. 2a), whereas the level of S. aureus diminished approximately 104-fold in this time period (Fig. 2c). The highest starting concentrations of P. aeruginosa increased at every time-point, the lower concentrations remained at a steady level (1021–1022 CFU/ml), whereas the lowest concentrations decreased 105 times after day 4 (Fig. 2d).
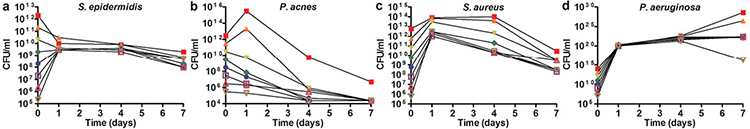
Fig. 2. Effect of the inoculum concentration on bacterial survival. (a) S. epidermidis, (b) P. acnes, (c) S. aureus, and (d) P. aeruginosa inoculated on the model in different concentrations ranging from 105 to 1012 colony-forming units (CFU)/ml and analysed on days 1, 4 and 7 by CFU counting. Colours represent different inoculation concentrations. Lower starting concentrations of inoculated bacteria resulted in increased growth on day 1, except for the lower concentrations of P. acnes. After the first day, all S. epidermidis, S. aureus and P. acnes concentrations followed a similar growth pattern. The concentrations of P. aeruginosa follow the same growth pattern up to day 4, then on day 7 the pattern changes depend on the starting concentration. This figure represents 1 of 2 separate experiments.
To determine whether callus is really indispensable as a source of nutrients for bacteria, S. epidermidis and S. aureus (~109–1010 CFU/ml) were inoculated on the model with no callus incorporated. Bacteria still showed a limited ability to proliferate at day 1; however, no viable bacteria were detected on the model without callus after 3 days of culture (Fig. S21).
Strain-specific qPCR and propidium monoazide treatment optimization
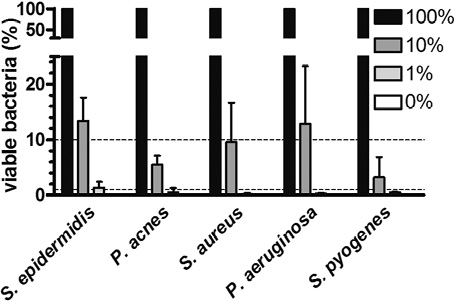
Counting CFU to analyse bacterial survival can be applied only when single strains are tested on the callus model, as it is difficult to distinguish between different bacterial colonies on blood agar plates. Therefore, strain-specific qPCR primers can be used to analyse mixed bacterial compositions when, for example, testing the efficacy of antimicrobial compounds on bacterial strains. However, during DNA isolation, gDNA from non-viable bacteria is also isolated and skews the qPCR data. PMA is able to penetrate the membrane of non-viable bacteria and, if exposed to light, binds covalently to gDNA. During isolation of gDNA and qPCR analysis, only unbound gDNA from viable cells can be isolated and amplified (33). The procedure for bacterial collection from the wells and subsequent analysis by PMA-qPCR analysis is shown in Fig. S31. To optimize the PMA treatment we studied the minimal period of light exposure that is effective against dead bacteria, without being harmful to viable cells. A 5 min light exposure resulted in a negligible effect on the viable bacteria compared with the no exposure condition (Fig. S41). The PMA procedure is effective to distinguish between viable and non-viable S. epidermidis, P. acnes, S. aureus, P. aeruginosa and S. pyogenes bacteria (Fig. S51). Subsequently, a ratio of viable and non-viable bacteria was made for all selected strains in order to validate the PMA treatment. Mixtures of viable and non-viable bacteria were exposed to PMA and light for 5 min, followed by gDNA isolation and qPCR analysis using Broad Range Universal (BRU) 16S rRNA gene primers (Table SI1). Using this PMA-qPCR protocol we could reproduce the viable/non-viable cells ratios as generated in advance (Fig. 3).
Establishing a “minimal” in vitro microbiome on the callus model
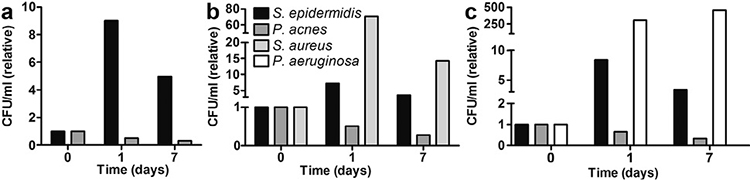
Using the callus model and a strain-specific PMA-qPCR as a read-out, we aimed to create a “minimal” microbiome in vitro. We used S. epidermidis and P. acnes strains, the 2 most common and abundant representatives of the Firmicutes and Actinobacteria, to create the “minimal” microbiome. Both skin commensals were inoculated together on the model (108 CFU/ml) and analysed on days 1 and 7 by PMA-qPCR using strain-specific primers (Table SI1). CFU/ml were calculated based on the Ct values using the calibration curve (Fig. S61). S. epidermidis showed the same growth curve (Fig. 4a; up on day 1, and down again on day 7) as when cultured as a single species on the model (Fig. 2a). P. acnes also showed the same growth curve in a mixture as cultured as a single species on the model (Fig. 2b).
Next, we investigated whether the addition of a pathogen had an effect on the minimal microbiome on the model system. S. aureus (108 CFU/ml) was added to both commensals on the model (Fig. 4b), but this had no quantifiable effect on the growth of S. epidermidis and P. acnes. Moreover, S. aureus showed the same growth curve as when cultured as a single species on the model. However, S. aureus cultured on the model (Fig. 2c) grew faster and to higher levels on the first day (~log4 scale), than when they were cultured together with S. epidermidis and P. acnes (~log2 scale, Fig. 4b). On day 7, comparable levels of S. aureus were observed in both experiments. The addition of P. aeruginosa (108 CFU/ml) to both commensals also did not lead to growth pattern-related changes in the minimal microbiome (Fig. 4c); however, P. aeruginosa, like S. aureus, showed a decreased proliferation rate when cultured in the presence of S. epidermidis and P. acnes.
Application of complete human skin microbiomes to the callus model
In vivo microbiomes of the lower back of 2 healthy volunteers (HV), obtained by skin swabbing, were cultured for one week on the callus model. The lower back was selected because of the large diversity of bacteria (10). For analysis, PMA-Illumina sequencing was applied. The Staphylococcus genus is the most abundant, other genera present include Corynebacterium, Propionibacterium, and Pseudomonas. The microbiota composition remained fairly constant over the week, except for the increased relative abundance of Corynebacterium species in HV1 on day 7 (Fig. S7a1). Moreover, phylogenetic diversity (PD) whole tree analysis, a metric for alpha diversity also taking taxonomic distance into account, showed that the bacterial alpha diversity remained constant over the 7 days of analysis (Fig. S7b1).
Efficacy of tetracycline on the callus model
To test whether our model is suitable to examine antibacterial properties of compounds, we tested the efficacy of tetracycline. The results were compared with the minimal inhibitory concentration (MIC) found in related experiments performed in Mueller Hinton culture medium (2 µg/ml for S. aureus and 64 µg/ml for S. epidermidis). S. epidermidis and S. aureus were cultured in medium and on our model for 24 h together with the skin antibiotic tetracycline. The efficacy of tetracycline was determined by CFU counting. We demonstrated that S. aureus is sensitive to tetracycline compared with S. epidermidis (Fig. 5a).
Bioluminescent bacteria for high-throughput analysis in the callus model
In order to create a model suitable for high-throughput screening, we down-scaled the model to 96-well plates and used bioluminescent S. aureus (Xen36). Different inoculum concentrations of the strain were applied to the model with or without the addition of 2% callus suspension (Fig. 5b and c). The different inoculum concentrations of Xen36 all reached the same number of bacteria on day 1, except for the highest inoculum concentration. This trend is similar to the growth of non-luminescent S. aureus in 24-well plates (Fig. 2c); the lower concentrations more rapidly than the higher concentrations. The bacteria showed no growth when the model was used without callus.
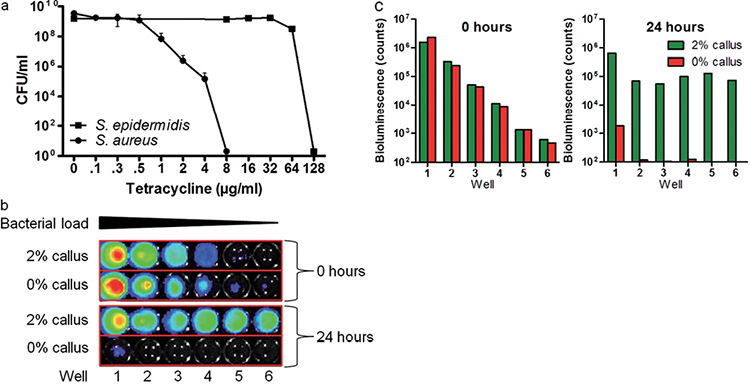
Fig. 5. Possible applications and usability of the callus model. (a) Testing of antimicrobial compounds. S. epidermidis and S. aureus were exposed for 24 h to tetracycline in the callus model. On the callus model, in accordance with growth in medium, S. aureus is more vulnerable to tetracycline compared with S. epidermidis. Values represent the mean ± standard deviation of 2 experiments. (b) Applicability of bioluminescent bacteria for high-throughput analysis. Bioluminescence of Xen36 measured with In Vivo Imaging System (IVIS; PerkinElmer, Waltham, MA, USA) at 0 and 24 h. Dilutions of 10 were pipetted on the model with and without 2% callus suspension, sample number 1 being the highest concentration of Xen36 on the model. (c) Bioluminescence (counts) of Xen36 in (b), analysed with Living Image 3.1 software.
DISCUSSION
This paper describes a newly developed stratum corneum model to study skin microbiota in vitro. The model is simple, affordable and amenable to high-throughput upscaling, and can be applied to study the growth of a single bacterial strain, a mixture of bacteria or a complete human skin microbiome. The PMA-qPCR analysis method ensures that only DNA from viable bacteria is quantified. Moreover, the model can be used to study the dynamics of microbial communities, e.g. following invasion by pathogenic micro-organisms or the application of antimicrobial compounds.
The callus-based skin stratum corneum model has advantages for studying microbial growth compared with human constructed skin equivalents. These cellular models are incubated at 37°C, a temperature that strongly favours bacterial growth. This is, however, not in accordance with the actual temperature of 32°C typically present on exposed skin. The model presented here can be prepared in advance and stored at 4°C. It consists only of 3 components (agar, callus and bacteria), and no further expensive matrix and medium components are necessary. The bacteria are inoculated and grown on a dry surface at 32°C. Our model is primarily designed to study the survival and growth of bacteria on the stratum corneum of the skin. To study the interaction of these bacteria with “living” epidermal cells, in vitro skin equivalents are the preferred model. However, the addition of recombinant proteins that play a role in the normal epidermal host-defence system to our model might be a good alternative to study the effect of these molecules on the bacterial colonization/composition.
We demonstrated that the callus we used contains sufficient nutrients for the growth of the most relevant strains and even for an entire skin microbiome. From our data, we deduced that callus probably does not contain much sebum components, which are present in vivo on specific locations of the human skin (34). Therefore, bacteria, such as P. acnes, which live on sebaceous areas of the human skin, are able to survive for only one week on our model. The addition of sebaceous components in future experiments might improve the growth of P. acnes on our model. We have also shown that S. epidermidis and S. aureus are not able to survive for more than one day without callus (Fig. S21). However, it is not clear which nutrients from these dead corneocytes are required for bacterial growth. Certainly, lipids in the callus are important factors for the bacteria, as we observed less bacterial proliferation when delipidized callus was used in the model (data not shown).
We created a “minimal” microbiome on our model consisting of S. epidermidis and P. acnes, and added pathogens (S. aureus and P. aeruginosa) to both of these commensals. As our model is suitable for studying the survival/growth of these pathogens, the effects of possible treatments on the pathogen as well as the commensal microbiota could be examined.
Moreover, we demonstrated that a complete in vivo skin microbiome can be inoculated on our model. Importantly, the bacterial diversity was relatively stable over a period of 7 days (bacterial alpha diversity remained constant, as shown in Fig. S7b1). Due to the low inoculation concentration, the in vivo microbiome was able to increase a number of log-scales, but this did not strongly affect the bacterial diversity and composition, except for the number of Propionibacterium spp., which decreased over time, probably due to a lack of sebum or lipid components in callus, as discussed above. Furthermore, we observed the presence of Pseudomonas species, which were infrequent to absent in our previous study (10). However, high levels of Pseudomonas on human skin were also reported by others (35). Our skin microbiome has a high degree of interpersonal variation and therefore it might be coincidental that the 2 samples in the present study contain higher Pseudomonas levels than the individuals in our previous studies. Alternatively, it is possible that the Pseudomonas sequences are derived from contaminations in aqueous solutions from the DNA isolation kit. However, the microbiome samples on day 7 do not contain high levels of Pseudomonas species. As the microbial DNA from these samples is isolated with the same kit, contamination is unlikely.
One of the main advantages of the in vitro skin stratum corneum model described here is the ability to control the proliferation rates of bacteria applied to the model. When we lowered the amount of inoculum, the bacteria showed increased growth at the first day (except for P. acnes) (Fig. 2). This results in a more dynamic, infection-like model, suitable for testing antibiotics. As an example we used tetracycline to evaluate its antimicrobial properties on the model; the MIC value of tetracycline in medium is 2 µg/ml for S. aureus and 64 µg/ml for S. epidermidis (not sensitive). Comparable values are found when S. epidermidis and S. aureus are exposed to tetracycline on the model. We therefore concluded that this model can be used to assess the antibacterial effects of antibiotics and antimicrobial proteins.
Furthermore, we down-scaled our model to a 96-well format, using bioluminescent bacteria. Bioluminescent bacteria (S. aureus Xen36 strain) could be detected on the model in a rapid and non-laborious way. The survival trend of the bioluminescent Xen36 strain is similar to the non-luminescent S. aureus strain that we used in 24-well plates (Fig. 2c), suggesting that our model can be used as a high-throughput system.
Possible applications of the stratum corneum model described here include intervention experiments to change disease-related microbiota compositions; for example, by prebiotics, probiotics or targeted antibiotics. In conclusion, we postulate that the in vitro skin stratum corneum model presented here will contribute to the understanding of diseases linked to bacterial colonization and/or infection.
ACKNOWLEDGEMENTS
This study is funded by The European Union and the Dutch provinces Gelderland and Overijssel (GO EFRO 2007-2013), by a grant from the Dutch Burns Foundation (P11.02), and by a personal fellowship (to PZ) from the ZonMw/NWO-ALW program Enabling Technologies, 2013.
The authors declare no conflicts of interest.

1http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-2401
REFERENCES
