Anna Rostedt Punga1, Annika Eriksson1 and Mohammad Alimohammadi2,3
1Institute of Neuroscience, Department of Clinical Neurophysiology, Uppsala University, 2Department of Medical Sciences, Uppsala University, and 3Department of Dermatology and Venereology, Uppsala University Hospital, Uppsala, Sweden
Despite the extensive use of botulinum toxin A (BoNTA) in medical and cosmetic treatments, the potential spreading of BoNTA to surrounding tissues remains unknown. A patient with hemifacial paralysis upon blepharospasm treatment with low dose of BoNTA, prompted us to investigate the spreading effect. A randomised, double-blind study was conducted in which 5 healthy women (33–52 years) were treated with different doses of onabotulinum toxin unilaterally in the corrugator muscle. Parameters of efficacy and diffusion (CMAP; EMG and jitter analysis) in both glabellar and frontalis muscles were assessed at baseline, 2 and 4 weeks following BoNTA injection. CMAP of the treated glabellar muscles was reduced to approximately 40% in all dose groups. Additionally, contralateral CMAP reduction was observed in 3 of 5 subjects. These data confirm regional diffusion of BoNTA in facial muscle application, which raises question on the reliability of split-face models in BoNTA studies. Key words: botulinum toxin; glabellar muscles; frontalis; CMAP; split-face.
Accepted Mar 11, 2015; Epub ahead of print Mar 13, 2015
Acta Derm Venereol 2015; 95:
Mohammad Alimohammadi, MD, PhD, Department of Medical Sciences, Uppsala University, University Hospital, SE-751 85 Uppsala, Sweden. E-mail: mohammad.alimohammadi@medsci.uu.se
Botulinum toxin type A (BoNTA) is one of the most potent neurotoxins, produced by the anaerobic bacteria Clostridium botulinum. BoNTA acts to inhibit the release of presynaptic actylcholine (ACh) from the neuromuscular junction (NMJ), through selective proteolysis of the synaptic protein SNAP-25 (1). The toxin has been approved for various medical and cosmetic applications, e.g. medical treatment of blepharospasm or cosmetic treatment of glabellar frown lines caused by tension in the glabellar muscles. BoNTA injection into these facial muscles results in muscle paralysis and hence diminished blepharospasm or glabellar frown lines.
Although treatment with BoNTA is considered safe, previous reports have demonstrated that BoNT can diffuse to muscles surrounding the treatment region and, in a dose-dependent manner, result in complications such as dysphagia (2). Some of the studies for cosmetic trials of BoNTA are designed in a split-face manner without any controls for the regional diffusion of the toxin to the contralateral side of the face (3, 4). However, it is known that BoNTA can indeed spread to contralateral facial muscles (5), which could influence the interpretation of the split-face model. Despite this, split-face studies have been applied to determine the effect of different BoNTA over time (6–8).
We have previously reported the correlation of neurophysiological parameters for assessment of dose response effect of BoNTA (9). Independent of our study, a patient case with an adverse event related to BoNTA regional diffusion, came to our knowledge in the neurology clinic. The aim of this prospective pilot study was to evaluate whether unilateral injection of BoNTA in the glabellar muscles results in regional diffusion to the surrounding and contralateral facial muscles.
MATERIAL AND METHODS
Ethics
The patient with adverse event signed an informed consent form for use of clinical photographs in publication purpose. This randomised neurophysiological study was approved by the Regional Ethical Review Board of Uppsala (Dnr: 2011/247) and the Medical Products Agency (EudraCT nr: 2011-004636-66). All subjects were enrolled after written informed consent.
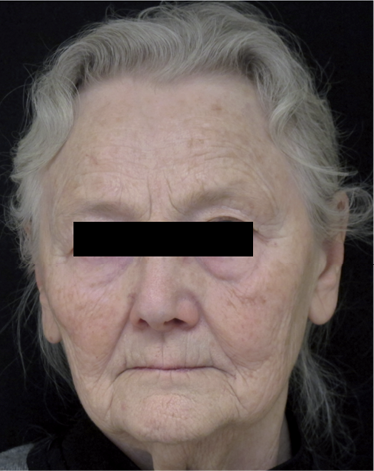
Patient case. An 80-year-old woman with blepharospasm was treated with 4 U of abotulinumtoxin A (Dysport®) in the left orbicularis oculi muscle. Eight days post-injection, the patient developed a left-sided hemi-facial paralysis, with inability to close her eyes tightly and drop in the left corner of the mouth. After a normal MRI of the brain, including the brain stem, she was diagnosed with peripheral facial nerve palsy as an adverse event to the injected BoNTA. The facial nerve palsy persisted at 4 weeks examination post-injection (Fig. 1). At the first neurophysiological evaluation, 10 days post-injection; EMG findings indicated acute partial denervation in the left orbicularis oris muscle. There was no suspicion that human factors in dilution and administration of the BoNTA could explain this adverse event. After 4 weeks a new electromyography (EMG) examination revealed slight signs of reinnervation in the left orbicularis oris muscle, despite persisting signs of the initial denervation.
Participants
Five healthy women, aged 33–52 years, were enrolled. Inclusion criteria were visible glabellar frown lines at rest. Exclusion criteria were diagnosis or signs of any neuromuscular disorder (including Myasthenia Gravis), allergy to BoNTA, previous BoNTA injection, plastic surgery or implants in the facial area, ongoing infection, systemic disease or inflammation in proximity to the injection areas, earlier unspecific severe allergic reactions, known coagulation disorder, medication with anticoagulant medication or muscle relaxants as well as pregnancy or lactation.
Study design
This study was designed as randomised double-blind where the evaluating neurophysiologist (ARP) and the study subjects were blinded to the given substance and dose until after the study was finalised, at 4 weeks following the initial injection. The neurophysiological and photographical base line examinations were performed before the intramuscular injection of onabotulinumtoxin A (Vistabel®). A dermatologist (MA) randomised the study subjects in 3 groups. The same injection volume, 0.1 ml, was given to all subjects in 2 unilateral standardised injection points in the corrugator muscle, using EMG guidance. Individuals in group 1 (n = 1) received 1.25 U/0.1 ml injection at each point (total 2.5 U); group 2 (n = 3) received 2.5 U/ 0.1 ml injection (total 5 U); Group 3 (n = 1) received 5 U/ 0.1 ml injection (total 10 U).
Neurophysiological examinations and parameters
All neurophysiological examinations were made on Keypoint equipment (Medtronic®).
Motor neurography with surface electrodes for stimulation of the temporal branch of the facial nerve (4 cm lateral to the eye) and recording over the corrugator and frontalis muscles. The compound motor action potential (CMAP) was defined with the main measures amplitude and area. The procedure was performed bilaterally.
Quantitative electromyography (EMG): A concentric facial EMG electrode (30G; 25 × 0.33 mm; Alpine Biomed, Skovlunde, Denmark) was placed in the corrugator supercilii muscle (0, 2, 4 weeks) and frontalis muscle (4 weeks) and analysed spontaneous activity (indicating ongoing denervation), motor unit potentials (MUPs) at slight voluntary activation and maximal muscle contraction (interference pattern).
Concentric needle jitter analysis (CNE): A disposable concentric facial EMG needle electrode (10) (30G; 25 × 0.33 mm; Alpine Biomed) was inserted in the middle muscle belly of the contralateral frontalis muscle. The jitter (µs) was expressed as the mean consecutive difference (MCD) of ≥ 20 analysed potential pairs (11) and this is the most sensitive parameter to measure disturbed neuromuscular transmission. Filter settings were set to 1000 Hz–10 kHz.
Photodocumentation of glabellar frown lines and subject diary
The study subjects were placed between 2 fluorescent strip lamps that were fixed in the ceiling, similar at all follow-up visits. A Nikon D5000 with fixed 105 mm Macro objective was used for the photo documentation. The glabellar area was photographed in 3 different modes: 1) relaxed mode 2) frontalis muscle contraction 3) maximal glabellar complex contraction. Glabellar frown lines in the relaxed mode could be assessed on the photos pre- and post-treatment. The pre-treatment glabellar lines could be graded based on a 4-point clinical severity scale from minimum 0 points to maximal 3 points (12). Additionally, study subjects were asked to document experienced adverse events.
Statistical methods
For each subject an absolute change and a percentage change was calculated relating post-treatment values to baseline (week 0). As the primary aim of this study was to assess the regional diffusion of the BoNTA in a pilot study, only a small number of individuals were included and statistical analysis not applicable between dose groups. The only statistical evaluations were performed based on within-subject changes on CNE where the hypothesis was that the true mean of change was 0 (1-sample t-test). A p-value ≤ 0.05 was considered significant.
RESULTS
Clinical characteristics and aesthetic treatment results
All 5 enrolled women were naive to previous BoNTA treatment. None of them met any exclusion criteria, confirmed by normal neurological examination as well as normal CNE analysis of neuromuscular transmission. All subjects were examined at 0, 2 and 4 weeks. One of the subjects (in the 10-U group) experienced adverse event consisting of ipsilateral lowering of the frontalis muscle. All patients had initial glabellar frown line scores graded 0 to 2. Representative photos are shown (Fig. 2).
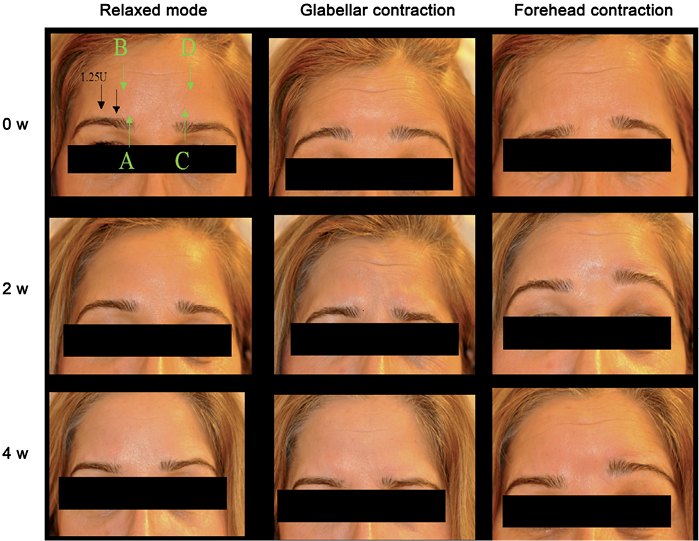
Fig. 2. Study design and photography of a representative study subject (subject 1 in Table I). Black arrows indicate injection points for the treatment. This study subject was treated with 1.25 units of onabotulinum toxin A (Vistabel®) at each injection site. Green arrows indicate the area/muscle where the CMAP was recorded. Labelling of the green arrows (A, B, C, D) correspond to the same panels seen in Fig. S11.
Muscle paralysis of the treated glabellar muscle as well as the ipsilateral frontalis muscle
Initial CMAP ranged from amplitude of 0.9–1.4 mV of the left and 0.8–1.5 mV of the right corrugator supercilii muscles. As expected, there was a substantial reduction of CMAP amplitude of the treated glabellar muscle in all subjects who received BoNTA between 0 and 2 weeks (Fig. S1A1). In most cases, the CMAP amplitude remained stable between the measurements at 2 and 4 weeks. In the frontalis muscle on the same side as the treated glabellar muscle, initial CMAP ranged from 1.1 to 1.8 mV. There was a gradual decrease in CMAP amplitude from week 0 to week 2 (~ 40% reduction; Table I) and further at 4 weeks (~ 50% reduction; Fig. S1B1).
Table I. Remaining amplitude of the compound motor action potential (CMAP) at 4 weeks following treatment of the unilateral corrugator muscle with botulinum toxin A, presented as percentage of the baseline value. Using EMG guidance, 2 injections of onabotulinumtoxin (Vistabel®) were given unilaterally in the corrogator muscle
|
Study subject |
Treated glabella side |
Dose/Inj (U) |
CMAP in the treated corrugator, % |
CMAP in the same side frontalis muscle, % |
CMAP in the contralateral corrugator, % |
CMAP in the contralateral frontalis muscle, % |
|
1 |
Right |
1.25 U |
40 |
58 |
91 |
100 |
|
2 |
Left |
2.5 U |
40 |
63 |
140 |
130 |
|
3 |
Right |
2.5 U |
50 |
50 |
55 |
100 |
|
4 |
Right |
2.5 U |
33 |
54 |
120 |
125 |
|
5 |
Right |
5 U |
38 |
50 |
78 |
80 |
EMG confirmed presence of abnormal spontaneous activity (fibrillations and/or positive sharp waves) due to pharmacological denervation at 4 weeks, both in the treated corrugator muscles and in the frontalis muscle on the same side in the subjects receiving 5 U or 10 U in the ipsilateral corrugator muscle, along with clearly reduced interference pattern upon maximal voluntary activity.
Diffusion effects of BoNTA in the contralateral facial muscles
The CMAP amplitude of the contralateral corrugator muscle was reduced in 3 subjects (Fig. S1C1), whereas the amplitude remained unchanged in one subject and slightly increased in one subject. In the contralateral frontalis muscle, CMAP amplitude decreased in 3 subjects (Figs S1D and S21) whereas it remained unaltered in 2 subjects.
Jitter analysis, in order to assess neuromuscular transmission failure, in the contralateral frontalis muscle at baseline, before BoNTA injection, revealed a mean jitter value of 28 µs (range 25–32 µs). Two weeks after the injection, the mean jitter value was significantly increased to 34 µs (range 27–39; p = 0.05). Blocking of neuromuscular transmission was present in approximately 5% of fibre pairs in 3 out of 5 subjects. This indicates that injection of BoNTA in the glabellar muscles on one side diffuses to the contralateral frontalis muscle and impairs the neuromuscular transmission.
DISCUSSION
Although treatments with BoNTA are considered relatively safe, regional diffusion of the toxin may cause benign as well as serious adverse events. Dose, volume and subsequent concentration of BoNT are parameters that all could influence diffusion and spreading of BoNT (13). In this report, we start to present a typical patient case with a benign but potentially serious adverse event, since regional diffusion of BoNTA can also cause bulbar muscle paralysis, which is potentially life threatening. In the presented patient case, EMG findings supported denervation in the facial nerve innervated orbicularis oculi muscle on the same side of the face as the injection site. Although this is not an impossible scenario, considering that the zygomatic branch of the facial nerve to some extent interconnects the ocular and buccal muscles, it is somewhat remarkable that this adverse event was caused by such a low dose of abotolinum toxin (4 U).
One of the most common and growing applications of BoNTA is the use in cosmetic treatments. As the marketing potential for the substance is lucrative, studies are performed to compare different preparations of the substance. Split-face models are often employed as an approach to compare different BoNTA formulations in aesthetic dermatological studies, where one preparation is injected on one side of the forehead and another preparation is injected in muscles on the contralateral side of the face. In these split-face models, the contralateral muscle activity is used as control in assessing the pharmacodynamics as well as the clinical attributes of the unilaterally injected BoNTA. Considering that different BoNTA preparations possess unique pharmacodynamic properties (14), this could however result in different diffusion properties of each toxin and bias the interpretation of the effect for each substance.
In a recent report, we demonstrated that CMAP in the facial muscles is a sensitive measure of muscle paralysis, especially in the glabellar area (9). In accordance with this recent study, the CMAP of the injected unilateral corrugator muscle decreased significantly as a consequence of the direct muscle paralysis. This is the desired cosmetic effect to reduce glabellar frown lines. Nevertheless, common experience from aesthetic treatments with BoNTA is that treatment of the glabellar muscles also results in a compensatory lift of the lateral aspects of the eyebrows, indicating a paralysis of the central parts of the frontalis muscle. Intriguingly, our CMAP data in the ipsilateral frontalis muscle demonstrate that the extent of muscle paralysis in the frontalis muscle is not considerably less than in the injected corrugator supercilii muscle. This is hence the neurophysiological evidence for a clinically well-known aesthetic effect of glabellar treatment on the lateral aspects of the eyebrows. Regarding the effects of BoNTA in the contralateral facial muscles, we observed reduced CMAP in the contralateral frontalis and/or corrugator muscles in 3 out of 5 subjects. However, in all subjects, independent of the injected dose of onabotulinumtoxin A, we observed a significantly increased jitter as measured by CNE. These data indicate disturbed neuromuscular transmission in the contralateral frontalis muscle, with no other explanation than a diffusion effect of BoNTA. Due to the measured spreading effects of BoNTA to the contralateral side of the face, the current study questions the reliability of split-face models in the glabellar area.
In conclusion, this pilot study provides objective evidence for diffusion of BoNTA to the contralateral facial muscles upon unilateral glabellar treatment. These data imply that one should be careful when interpreting the results from “split-face” models that characterise and compare different BoNTA preparations. From a drug safety perspective, the results of this study highlight that the toxin actually diffuses to regional parts of the treated area encouraging future studies to determine whether BoNTA treatments may have any long-term adverse events on organs with proximity to the treated area.
Acknowledgements
The authors are grateful to Jan-Erik Anheller for monitoring the study.
Conflict of interest: The study was an investigator-initiated study and the manuscript was prepared solely by the authors. MOA and ARP have received consulting fees from Q-MED AB.

1http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-2093
REFERENCES
